Discovery of antibacterial cyclic peptides that inhibit the ClpXP protease
- PMID: 17600141
- PMCID: PMC2203360
- DOI: 10.1110/ps.072933007
Discovery of antibacterial cyclic peptides that inhibit the ClpXP protease
Abstract
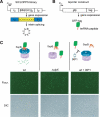
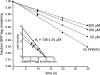
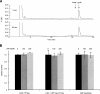
A method to rapidly screen libraries of cyclic peptides in vivo for molecules with biological activity has been developed and used to isolate cyclic peptide inhibitors of the ClpXP protease. Fluorescence activated cell sorting was used in conjunction with a fluorescent reporter to isolate cyclic peptides that inhibit the proteolysis of tmRNA-tagged proteins in Escherichia coli. Inhibitors shared little sequence similarity and interfered with unexpected steps in the ClpXP mechanism in vitro. One cyclic peptide, IXP1, inhibited the degradation of unrelated ClpXP substrates and has bactericidal activity when added to growing cultures of Caulobacter crescentus, a model organism that requires ClpXP activity for viability. The screen used here could be adapted to identify cyclic peptide inhibitors of any enzyme that can be expressed in E. coli in conjunction with a fluorescent reporter.
Figures

Similar articles
-
Identification of ClpP substrates in Caulobacter crescentus reveals a role for regulated proteolysis in bacterial development.Mol Microbiol. 2013 Jun;88(6):1083-92. doi: 10.1111/mmi.12241. Epub 2013 May 7. Mol Microbiol. 2013. PMID: 23647068 Free PMC article.
-
Polar Localization Hub Protein PopZ Restrains Adaptor-Dependent ClpXP Proteolysis in Caulobacter crescentus.J Bacteriol. 2018 Sep 24;200(20):e00221-18. doi: 10.1128/JB.00221-18. Print 2018 Oct 15. J Bacteriol. 2018. PMID: 30082457 Free PMC article.
-
Lassomycin, a ribosomally synthesized cyclic peptide, kills mycobacterium tuberculosis by targeting the ATP-dependent protease ClpC1P1P2.Chem Biol. 2014 Apr 24;21(4):509-518. doi: 10.1016/j.chembiol.2014.01.014. Epub 2014 Mar 27. Chem Biol. 2014. PMID: 24684906 Free PMC article.
-
New peptides isolated from Lyngbya species: a review.Mar Drugs. 2010 Jun 9;8(6):1817-37. doi: 10.3390/md8061817. Mar Drugs. 2010. PMID: 20631872 Free PMC article. Review.
-
Protease regulation and capacity during Caulobacter growth.Curr Opin Microbiol. 2016 Dec;34:75-81. doi: 10.1016/j.mib.2016.07.017. Epub 2016 Aug 18. Curr Opin Microbiol. 2016. PMID: 27543838 Free PMC article. Review.
Cited by
-
Validation of the essential ClpP protease in Mycobacterium tuberculosis as a novel drug target.J Bacteriol. 2012 Feb;194(3):663-8. doi: 10.1128/JB.06142-11. Epub 2011 Nov 28. J Bacteriol. 2012. PMID: 22123255 Free PMC article.
-
Transcription of clpP is enhanced by a unique tandem repeat sequence in Streptococcus mutans.J Bacteriol. 2009 Feb;191(3):1056-65. doi: 10.1128/JB.01436-08. Epub 2008 Dec 1. J Bacteriol. 2009. PMID: 19047352 Free PMC article.
-
Cyclic Peptides: Promising Scaffolds for Biopharmaceuticals.Genes (Basel). 2018 Nov 16;9(11):557. doi: 10.3390/genes9110557. Genes (Basel). 2018. PMID: 30453533 Free PMC article. Review.
-
Intein-mediated backbone cyclization of entolimod confers enhanced radioprotective activity in mouse models.PeerJ. 2018 Jun 18;6:e5043. doi: 10.7717/peerj.5043. eCollection 2018. PeerJ. 2018. PMID: 29938138 Free PMC article.
-
Ribosomal Synthesis of Natural-Product-Like Bicyclic Peptides in Escherichia coli.Chembiochem. 2015 Sep 21;16(14):2011-6. doi: 10.1002/cbic.201500179. Epub 2015 Aug 6. Chembiochem. 2015. PMID: 26179106 Free PMC article.
References
-
- Abel-Santos E., Scott, C.P., and Benkovic, S.J. 2003. Use of inteins for the in vivo production of stable cyclic peptide libraries in E. coli . Methods Mol. Biol. 205: 281–294. - PubMed
-
- Brotz-Oesterhelt H., Beyer, D., Kroll, H.P., Endermann, R., Ladel, C., Schroeder, W., Hinzen, B., Raddatz, S., Paulsen, H., Henninger, K., et al. 2005. Dysregulation of bacterial proteolytic machinery by a new class of antibiotics. Nat. Med. 11: 1082–1087. - PubMed
-
- Chen B., Guo, Q., Guo, Z., and Wang, X. 2003. An improved activity assay method for arginine kinase based on a ternary heteropolyacid system. Tsinghua Sci. Technol. 8: 422–427.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

