Effect of Asp69 and Arg310 on the pK of His68, a key catalytic residue of adenylosuccinate lyase
- PMID: 17600142
- PMCID: PMC2203377
- DOI: 10.1110/ps.072927207
Effect of Asp69 and Arg310 on the pK of His68, a key catalytic residue of adenylosuccinate lyase
Abstract
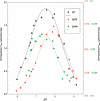
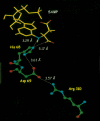
Adenylosuccinate lyase (ASL) of Bacillus subtilis contains three conserved histidines, His(68), His(89), and His(141), identified by affinity labeling and site-directed mutagenesis as critical to the intersubunit catalytic site. The pH-V(max) profile for wild-type ASL is bell-shaped (pK (1) = 6.74 and pK (2) = 8.28). Only the alkaline side changes with temperature, characteristic of histidine pKs. To identify determinants of pK (2) in the enzyme-substrate complex, we replaced residues at two positions close to His(68) (but not to His(89) or His(141)) in the structure. Compared with the specific activity of 1.75 mumol adenylosuccinate reacting/min/mg of wild-type enzyme at pH 7.0, mutant enzymes D69E, D69N, R310Q, and R310K exhibit specific activities of 0.40, 0.04, 0.00083, and 0.10, respectively. While D69E has a K (m) for adenylosuccinate similar to that of wild-type ASL, D69N and R310K exhibit modest increases in K (m), and R310Q has an 11-fold increase in K (m). The mutant enzymes show no significant change in molecular weight or secondary structure. The major change is in the pH-V(max) profile: pK (2) is 8.48 for the D69E mutant and is decreased to 7.83 in D69N, suggesting a proximal negative charge is needed to maintain the high pK of 8.28 observed for wild-type enzyme and attributed to His(68). Similarly, R310Q exhibits a decrease in its pK (2) (7.33), whereas R310K shows little change in pK (2) (8.24). These results suggest that Asp(69) interacts with His(68), that Arg(310) interacts with and orients the beta-carboxylate of Asp(69), and that His(68) must be protonated for ASL to be active.
Figures

References
-
- Bradford M.M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254. - PubMed
-
- Bridger W.A. and Cohen, L.H. 1968. The kinetics of adenylosuccinate lyase. J. Biol. Chem. 243: 644–650. - PubMed
-
- Brosius J.L. and Colman, R.F. 2000. A key role in catalysis for His89 of adenylosuccinate lyase of Bacillus subtilis . Biochemistry 39: 13336–13343. - PubMed
-
- Brosius J.L. and Colman, R.F. 2002. Three subunits contribute amino acids to the active site of tetrameric adenylosuccinate lyase: Lys268 and Glu275 are required. Biochemistry 41: 2217–2226. - PubMed
-
- Frey P.A. 1989. Chiral phosphorothioates: Stereochemical analysis of enzymatic substitution at phosphorous. Adv. Enzymol. Relat. Areas Mol. Biol. 62: 119–201. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

