Fas-induced pulmonary apoptosis and inflammation during indirect acute lung injury
- PMID: 17600273
- PMCID: PMC1994224
- DOI: 10.1164/rccm.200611-1743OC
Fas-induced pulmonary apoptosis and inflammation during indirect acute lung injury
Abstract
Rationale: Indirect acute lung injury (ALI) is associated with high morbidity and mortality. No specific therapies have been developed, because the underlying pathophysiological processes remain elusive.
Objectives: To investigate the contribution of Fas-induced apoptotic and nonapoptotic/inflammatory signaling to the pathology of indirect ALI.
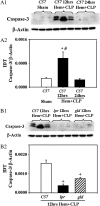
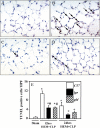
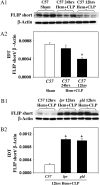
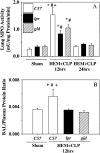
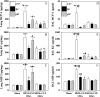
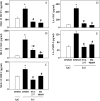
Methods: A mouse model of indirect ALI, induced by successive exposure to hemorrhagic shock and cecal ligation and puncture, was used. Quantification of active caspase-3 and the short splice variant of FLICE-inhibitory protein, (FLIP)short, was performed by Western blotting and immunohistochemistry, and cytokines/chemokines were assessed by cytometric bead array or ELISA. M30 immunostaining was done to evaluate epithelial cell apoptosis. Lung injury was assessed on the basis of myeloperoxidase activity, bronchoalveolar lavage protein, and lung histology.
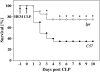
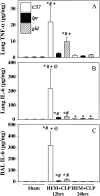
Measurements and main results: Twelve hours after insult, lung monocyte chemoattractant protein-1, keratinocyte-derived chemokine, macrophage inflammatory protein-2, IL-6, tumor necrosis factor-alpha, and caspase-3 were increased and FLIP(short) was decreased. Fas- and Fas ligand-deficient mice showed marked protection from lung inflammation and apoptosis and decreased ALI. This was associated with a 10-day survival benefit. Similarly, 4 hours after pulmonary instillation of Fas-activating antibody in vivo, lung chemokines were markedly elevated in background mice and, interestingly, to a similar degree in macrophage-deficient animals. Fas activation on lung epithelial cells in vitro led to chemokine production that was dependent on extracellular signal-regulated kinase.
Conclusions: Activation of apoptotic and nonapoptotic/inflammatory Fas signaling is an early important pathophysiological event in the development of indirect ALI after hemorrhagic shock and sepsis, in which lung epithelial cells appear to play a central role.
Figures




References
-
- Frutos-Vivar F, Nin N, Esteban A. Epidemiology of acute lung injury and acute respiratory distress syndrome. Curr Opin Crit Care 2004;10:1–6. - PubMed
-
- MacCallum NS, Evans TW. Epidemiology of acute lung injury. Curr Opin Crit Care 2005;11:43–49. - PubMed
-
- Brun-Buisson C, Minelli C, Bertolini G, Brazzi L, Pimentel J, Lewandowski K, Bion J, Romand JA, Villar J, Thorsteinsson A, et al. Epidemiology and outcome of acute lung injury in European intensive care units: results from the ALIVE Study. Intensive Care Med 2004;30:51–61. - PubMed
-
- Bersten AD, Edibam C, Hunt T, Moran J. Incidence and mortality of acute lung injury and the acute respiratory distress syndrome in three Australian states. Am J Respir Crit Care Med 2002;165:443–448. - PubMed
-
- Takeda S, Ishizaka A, Fujino Y, Fukuoka T, Nagano O, Yamada Y, Takezawa J. Time to change diagnostic criteria of ARDS: towards the disease entity-based subgrouping. Pulm Pharmacol Ther 2005;18:115–119. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

