The signaling pathway involved in neutrophil elastase stimulated MUC1 transcription
- PMID: 17600314
- PMCID: PMC2219551
- DOI: 10.1165/rcmb.2007-0072OC
The signaling pathway involved in neutrophil elastase stimulated MUC1 transcription
Abstract
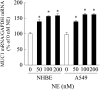
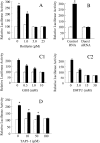
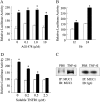
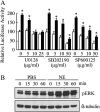
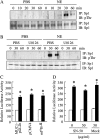
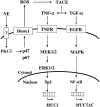
We previously reported that neutrophil elastase (NE) stimulated MUC1 gene expression in A549 lung epithelial cells through binding of Sp1 to the MUC1 promoter element. The current study was undertaken to elucidate the complete signaling pathway leading to Sp1 activation. Using a combination of pharmacologic inhibitors, dominant-negative mutant, RNA interference, and soluble receptor blocking techniques, we identified a protein kinase Cdelta (PKCdelta) --> dual oxidase 1 (Duox1) --> reactive oxygen species (ROS) --> TNF-alpha-converting enzyme (TACE) --> TNF-alpha --> TNF receptor (TNFR)1 --> extracellular signal-regulated kinase (ERK)1/2 --> Sp1 pathway as responsible for NE-activated MUC1 transcription. This cascade was identical up to the point of TACE with the signaling pathway previously reported for NE-stimulated MUC5AC production. However, unlike the MUC5AC pathway, TNF-alpha, TNFR1, ERK1/2, and Sp1 were unique components of the MUC1 pathway. Given the anti-inflammatory role of MUC1 during airway bacterial infection, up-regulation of MUC1 by inflammatory mediators such as NE and TNF-alpha suggests a crucial role for MUC1 in the control of excessive inflammation during airway bacterial infection.
Figures

Similar articles
-
Dual oxidase 1-dependent MUC5AC mucin expression in cultured human airway epithelial cells.Proc Natl Acad Sci U S A. 2005 Jan 18;102(3):767-72. doi: 10.1073/pnas.0408932102. Epub 2005 Jan 7. Proc Natl Acad Sci U S A. 2005. PMID: 15640347 Free PMC article.
-
TNF-alpha induces MUC1 gene transcription in lung epithelial cells: its signaling pathway and biological implication.Am J Physiol Lung Cell Mol Physiol. 2007 Sep;293(3):L693-701. doi: 10.1152/ajplung.00491.2006. Epub 2007 Jun 15. Am J Physiol Lung Cell Mol Physiol. 2007. PMID: 17575006
-
Neutrophil elastase induces MUC5AC mucin production in human airway epithelial cells via a cascade involving protein kinase C, reactive oxygen species, and TNF-alpha-converting enzyme.J Immunol. 2005 Sep 15;175(6):4009-16. doi: 10.4049/jimmunol.175.6.4009. J Immunol. 2005. PMID: 16148149
-
TACE: a new target in epidermal growth factor receptor dependent tumors.Differentiation. 2007 Nov;75(9):800-8. doi: 10.1111/j.1432-0436.2007.00198.x. Epub 2007 Jul 2. Differentiation. 2007. PMID: 17608729 Review.
-
MUC1: a novel metabolic master regulator.Biochim Biophys Acta. 2014 Apr;1845(2):126-35. doi: 10.1016/j.bbcan.2014.01.001. Epub 2014 Jan 11. Biochim Biophys Acta. 2014. PMID: 24418575 Free PMC article. Review.
Cited by
-
ATP-mediated activation of the NADPH oxidase DUOX1 mediates airway epithelial responses to bacterial stimuli.J Biol Chem. 2009 Jun 26;284(26):17858-67. doi: 10.1074/jbc.M809761200. Epub 2009 Apr 21. J Biol Chem. 2009. PMID: 19386603 Free PMC article.
-
Combination of curcumin and bicalutamide enhanced the growth inhibition of androgen-independent prostate cancer cells through SAPK/JNK and MEK/ERK1/2-mediated targeting NF-κB/p65 and MUC1-C.J Exp Clin Cancer Res. 2015 May 15;34(1):46. doi: 10.1186/s13046-015-0168-z. J Exp Clin Cancer Res. 2015. PMID: 25971429 Free PMC article.
-
A novel mesenchymal stem cell-based regimen for acute myeloid leukemia differentiation therapy.Acta Pharm Sin B. 2023 Jul;13(7):3027-3042. doi: 10.1016/j.apsb.2023.05.007. Epub 2023 May 16. Acta Pharm Sin B. 2023. PMID: 37521858 Free PMC article.
-
MUC1 (CD227): a multi-tasked molecule.Cell Mol Life Sci. 2015 Dec;72(23):4475-500. doi: 10.1007/s00018-015-2014-z. Epub 2015 Aug 21. Cell Mol Life Sci. 2015. PMID: 26294353 Free PMC article. Review.
-
Higher susceptibility to experimental autoimmune encephalomyelitis in Muc1-deficient mice is associated with increased Th1/Th17 responses.Brain Behav Immun. 2013 Mar;29:70-81. doi: 10.1016/j.bbi.2012.12.004. Epub 2012 Dec 20. Brain Behav Immun. 2013. PMID: 23261777 Free PMC article.
References
-
- Hollingsworth MA, Batra SK, Qi WN, Yankaskas JR. MUC1 mucin mRNA expression in cultured human nasal and bronchial epithelial cells. Am J Respir Cell Mol Biol 1992;6:516–520. - PubMed
-
- Park H, Hyun SW, Kim KC. Expression of MUC1 mucin gene by hamster tracheal surface epithelial cells in primary culture. Am J Respir Cell Mol Biol 1996;15:237–244. - PubMed
-
- Gendler SJ. MUC1, the renaissance molecule. J Mammary Gland Biol Neoplasia 2001;6:339–353. - PubMed
-
- Lu W, Hisatsune A, Koga T, Kato K, Kuwahara I, Lillehoj EP, Chen W, Cross AS, Gendler SJ, Gewirtz AT, et al. Cutting edge: enhanced pulmonary clearance of Pseudomonas aeruginosa by Muc1 knockout mice. J Immunol 2006;176:3890–3894. - PubMed
-
- Kuwahara I, Lillehoj EP, Hisatsune A, Lu W, Isohama Y, Miyata T, Kim KC. Neutrophil elastase stimulates MUC1 gene expression through increased Sp1 binding to the MUC1 promoter. Am J Physiol Lung Cell Mol Physiol 2005;289:L355–L362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

