Astrocytic connexin distributions and rapid, extensive dye transfer via gap junctions in the inferior colliculus: implications for [(14)C]glucose metabolite trafficking
- PMID: 17600824
- PMCID: PMC2819729
- DOI: 10.1002/jnr.21376
Astrocytic connexin distributions and rapid, extensive dye transfer via gap junctions in the inferior colliculus: implications for [(14)C]glucose metabolite trafficking
Abstract
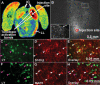
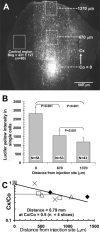
The inferior colliculus has the highest rates of blood flow and metabolism in brain, and functional metabolic activity increases markedly in response to acoustic stimulation. However, brain imaging with [1- and 6-(14)C]glucose greatly underestimates focal metabolic activation that is readily detected with [(14)C]deoxyglucose, suggesting that labeled glucose metabolites are quickly dispersed and released from highly activated zones of the inferior colliculus. To evaluate the role of coupling of astrocytes via gap junctions in dispersal of molecules within the inferior colliculus, the present study assessed the distribution of connexin (Cx) proteins in the inferior colliculus and spreading of Lucifer yellow from single microinjected astrocytes in slices of adult rat brain. Immunoreactive Cx43, Cx30, and Cx26 were heterogeneously distributed; the patterns for Cx43 and Cx 30 differed and were similar to those of immunoreactive GFAP and S100beta, respectively. Most Cx43 was phosphorylated in resting and acoustically stimulated rats. Dye spreading revealed an extensive syncytial network that included thousands of cells and perivasculature endfeet; with 8% Lucifer yellow VS and a 5-min diffusion duration, about 6,100 astrocytes (range 2,068-11,939) were labeled as far as 1-1.5 mm from the injected cell. The relative concentration of Lucifer yellow fell by 50% within 0.3-0.8 mm from the injected cell with a 5-min diffusion interval. Perivascular dye labeling was readily detectable and often exceeded dye levels in nearby neuropil. Thus, astrocytes have the capability to distribute intracellular molecules quickly from activated regions throughout the large, heterogeneous syncytial volume of the inferior colliculus, and rapid trafficking of labeled metabolites would degrade resolution of focal metabolic activation.
Figures





References
-
- Ackermann RF, Lear JL. Glycolysis-induced discordance between glucose metabolic rates measured with radiolabeled fluorodeoxyglucose and glucose. J Cereb Blood Flow Metab. 1989;9:774–785. - PubMed
-
- Adachi K, Cruz NF, Sokoloff L, Dienel GA. Labeling of metabolic pools by [6-14C]glucose during K+-induced stimulation of glucose utilization in rat brain. J Cereb Blood Flow Metab. 1995;15:97–110. - PubMed
-
- Binmöller FJ, Müller CM. Postnatal development of dye-coupling among astrocytes in rat visual cortex. Glia. 1992;6:127–137. - PubMed
-
- Brightman MW. The brain's interstitial clefts and their glial walls. J Neurocytol. 2002;31:595–603. - PubMed
-
- Bruzzone R, White TW, Paul DL. Connections with connexins: the molecular basis of direct intercellular signaling. Eur J Biochem. 1996;238:1–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

