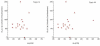Saliva soluble HLA as a potential marker of response to interferon-beta 1a in multiple sclerosis: a preliminary study
- PMID: 17601341
- PMCID: PMC1939839
- DOI: 10.1186/1742-2094-4-16
Saliva soluble HLA as a potential marker of response to interferon-beta 1a in multiple sclerosis: a preliminary study
Abstract
Objective: Potential surrogate markers of disease activity, including response to therapy, are particularly important in a neurological disorder such as multiple sclerosis (MS) which often has a fluctuating course. Based upon previous studies in our laboratory, we hypothesized that measurement of soluble HLA (sHLA) molecules class II in saliva of MS patients can serve as marker of therapeutic response to high dose interferon beta-1a.
Methods: We measured the expression patterns of sHLA-II in saliva in 17 patients with relapsing/remitting MS and compared the results to clinical course and brain MRI. For comparison purposes we also assayed the saliva sHLA-II levels in 53 normal control subjects. Solid phase ELISA was used for measurement of sHLA-I and sHLA-II concentrations at baseline and after three and six months of treatment with high dose interferon beta-1a (IFN beta-1a).
Results: The mean saliva sHLA-ll levels in MS patients was significantly higher than normal controls (354 +/- 42 unit/mL vs. 222 +/- 18 unit/mL, t= 8.16, p < 0.003). Comparison of saliva sHLA-II values before and after treatment with IFN beta-1a revealed a consistent increase in mean concentration. The increase in saliva sHLA-II values (354 +/- 42 unit/mL at baseline versus 821 +/- 86 unit/mL at 3 months and 776 +/- 63 unit/mL at 6 months, in unit/mL, p < 0.001 for both comparisons) was associated with a stable clinical course and a decline of the number of contrast-enhancing lesions on brain MRI. Comparison of the volume of T2-weighted lesions and the number of black holes on T1-weighted images did not reveal any significant changes (during pre-treatment versus post-treatment month 6) or any correlations with saliva sHLA-II levels. Saliva sHLA-I levels were not detectable.
Conclusion: Serial measurement of saliva sHLA-II may serve as a potential marker of therapeutic response to IFN beta-1a. Larger clinical studies involving more RRMS patients over longer periods of time are needed to further test the significance and value of saliva sHLA-II as an accurate marker of therapeutic response to beta-interferons.
Figures
Similar articles
-
Beneficial effect of interferon-beta 1b treatment in patients with relapsing-remitting multiple sclerosis is associated with an increase in serum levels of soluble HLA-I molecules during the first 3 months of therapy.J Neuroimmunol. 2004 Mar;148(1-2):206-11. doi: 10.1016/j.jneuroim.2003.12.002. J Neuroimmunol. 2004. PMID: 14975603 Clinical Trial.
-
Soluble HLA measurement in saliva and cerebrospinal fluid in Caucasian patients with multiple sclerosis: a preliminary study.J Neuroinflammation. 2005 Jun 2;2:13. doi: 10.1186/1742-2094-2-13. J Neuroinflammation. 2005. PMID: 15932635 Free PMC article.
-
Soluble HLA class I and class II molecules in relapsing-remitting multiple sclerosis: acute response to interferon-beta1a treatment and their use as markers of disease activity.Ann N Y Acad Sci. 2005 Jun;1051:111-20. doi: 10.1196/annals.1361.052. Ann N Y Acad Sci. 2005. PMID: 16126950
-
Comparison of fingolimod with interferon beta-1a in relapsing-remitting multiple sclerosis: a randomised extension of the TRANSFORMS study.Lancet Neurol. 2011 Jun;10(6):520-9. doi: 10.1016/S1474-4422(11)70099-0. Epub 2011 May 13. Lancet Neurol. 2011. PMID: 21571593 Clinical Trial.
-
CSF levels of soluble HLA-G and Fas molecules are inversely associated to MRI evidence of disease activity in patients with relapsing-remitting multiple sclerosis.Mult Scler. 2008 May;14(4):446-54. doi: 10.1177/1352458507085137. Epub 2008 Jan 21. Mult Scler. 2008. PMID: 18208868
Cited by
-
Soluble HLA peptidome: A new resource for cancer biomarkers.Front Oncol. 2022 Dec 22;12:1069635. doi: 10.3389/fonc.2022.1069635. eCollection 2022. Front Oncol. 2022. PMID: 36620582 Free PMC article. Review.
-
Relevance of Saliva Analyses in Terms of Etiological Factors, Biomarkers, and Indicators of Disease Course in Patients with Multiple Sclerosis-A Review.Int J Mol Sci. 2024 Nov 22;25(23):12559. doi: 10.3390/ijms252312559. Int J Mol Sci. 2024. PMID: 39684271 Free PMC article. Review.
-
Salivary biomarkers for the diagnosis and monitoring of neurological diseases.Biomed J. 2018 Apr;41(2):63-87. doi: 10.1016/j.bj.2018.03.004. Epub 2018 May 10. Biomed J. 2018. PMID: 29866603 Free PMC article. Review.
-
Biomarkers in Multiple Sclerosis: An Up-to-Date Overview.Mult Scler Int. 2013;2013:340508. doi: 10.1155/2013/340508. Epub 2013 Jan 22. Mult Scler Int. 2013. PMID: 23401777 Free PMC article.
-
Salivary Biomarkers: Future Approaches for Early Diagnosis of Neurodegenerative Diseases.Brain Sci. 2020 Apr 21;10(4):245. doi: 10.3390/brainsci10040245. Brain Sci. 2020. PMID: 32326227 Free PMC article. Review.
References
-
- Davies H, Pollard S, Calne R. Soluble HLA antigens in the circulation of liver graft recipients. Transplantation. 1989;47:524–527. - PubMed
-
- Adamashvili I, McDonald J, Fraser P, Milford E, Pressly T, Gelder F. Soluble class I HLA antigens in patients with rheumatoid arthritis and their families. J Rheumatol. 1995;22:1025–1031. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials