The plant pathogen Ralstonia solanacearum needs aerotaxis for normal biofilm formation and interactions with its tomato host
- PMID: 17601784
- PMCID: PMC1951909
- DOI: 10.1128/JB.00398-07
The plant pathogen Ralstonia solanacearum needs aerotaxis for normal biofilm formation and interactions with its tomato host
Abstract
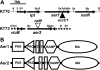
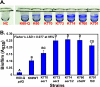
Ralstonia solanacearum is a soilborne pathogen that causes bacterial wilt of diverse plant species. To locate and infect host plant roots R. solanacearum needs taxis, the ability to move toward more favorable conditions. However, the specific signals that attract this pathogen were unknown. One candidate is aerotaxis, or energy taxis, which guides bacteria toward optimal intracellular energy levels. The R. solanacearum genome encodes two putative aerotaxis transducers. Cloned R. solanacearum aer1 and aer2 genes restored aerotaxis to an Escherichia coli aer mutant, demonstrating that both genes encode heterologously functional aerotaxis transducers. Site-directed mutants lacking aer1, aer2, or both aer1 and aer2 were significantly less able to move up an oxygen gradient than the wild-type parent strain; in fact, the aerotaxis of the aer mutants was indistinguishable from that of a completely nonmotile strain. Tomato plants inoculated with either the aer2 or the aer1/aer2 mutant had slightly delayed wilt disease development. Furthermore, the aer1/aer2 double mutant was significantly impaired in the ability to rapidly localize on tomato roots compared to its wild-type parent. Unexpectedly, all nonaerotactic mutants formed thicker biofilms on abiotic surfaces than the wild type. These results indicate that energy taxis contributes significantly to the ability of R. solanacearum to locate and effectively interact with its host plants.
Figures




References
-
- Alexandre, G., S. Greer-Phillips, and I. B. Zhulin. 2004. Ecological role of energy taxis in microorganisms. FEMS Microbiol. Rev. 28:113-126. - PubMed
-
- Allen, C., Y. Huang, and L. Sequeira. 1991. Cloning of genes affecting polygalacturonase production in Pseudomonas solanacearum. Mol. Plant-Microbe Interact. 4:147-154.
-
- Aravind, L., and C. P. Ponting. 1999. The cytoplasmic helical linker domain of receptor histidine kinase and methyl-accepting proteins is common to many prokaryotic signalling proteins. FEMS Microbiol. Lett. 176:111-116. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources

