The Arabidopsis MATE transporter TT12 acts as a vacuolar flavonoid/H+ -antiporter active in proanthocyanidin-accumulating cells of the seed coat
- PMID: 17601828
- PMCID: PMC1955721
- DOI: 10.1105/tpc.106.046029
The Arabidopsis MATE transporter TT12 acts as a vacuolar flavonoid/H+ -antiporter active in proanthocyanidin-accumulating cells of the seed coat
Abstract
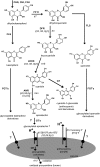
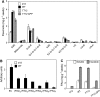
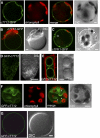
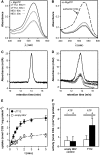
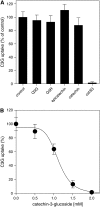
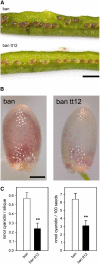
Phenotypic characterization of the Arabidopsis thaliana transparent testa12 (tt12) mutant encoding a membrane protein of the multidrug and toxic efflux transporter family, suggested that TT12 is involved in the vacuolar accumulation of proanthocyanidin precursors in the seed. Metabolite analysis in tt12 seeds reveals an absence of flavan-3-ols and proanthocyanidins together with a reduction of the major flavonol quercetin-3-O-rhamnoside. The TT12 promoter is active in cells synthesizing proanthocyanidins. Using translational fusions between TT12 and green fluorescent protein, it is demonstrated that this transporter localizes to the tonoplast. Yeast vesicles expressing TT12 can transport the anthocyanin cyanidin-3-O-glucoside in the presence of MgATP but not the aglycones cyanidin and epicatechin. Inhibitor studies demonstrate that TT12 acts in vitro as a cyanidin-3-O-glucoside/H(+)-antiporter. TT12 does not transport glycosylated flavonols and procyanidin dimers, and a direct transport activity for catechin-3-O-glucoside, a glucosylated flavan-3-ol, was not detectable. However, catechin-3-O-glucoside inhibited TT12-mediated transport of cyanidin-3-O-glucoside in a dose-dependent manner, while flavan-3-ol aglycones and glycosylated flavonols had no effect on anthocyanin transport. It is proposed that TT12 transports glycosylated flavan-3-ols in vivo. Mutant banyuls (ban) seeds accumulate anthocyanins instead of proanthocyanidins, yet the ban tt12 double mutant exhibits reduced anthocyanin accumulation, which supports the transport data suggesting that TT12 mediates anthocyanin transport in vitro.
Figures

Similar articles
-
MATE transporters facilitate vacuolar uptake of epicatechin 3'-O-glucoside for proanthocyanidin biosynthesis in Medicago truncatula and Arabidopsis.Plant Cell. 2009 Aug;21(8):2323-40. doi: 10.1105/tpc.109.067819. Epub 2009 Aug 14. Plant Cell. 2009. PMID: 19684242 Free PMC article.
-
The TRANSPARENT TESTA12 gene of Arabidopsis encodes a multidrug secondary transporter-like protein required for flavonoid sequestration in vacuoles of the seed coat endothelium.Plant Cell. 2001 Apr;13(4):853-71. doi: 10.1105/tpc.13.4.853. Plant Cell. 2001. PMID: 11283341 Free PMC article.
-
Arabidopsis pab1, a mutant with reduced anthocyanins in immature seeds from banyuls, harbors a mutation in the MATE transporter FFT.Plant Mol Biol. 2016 Jan;90(1-2):7-18. doi: 10.1007/s11103-015-0389-8. Epub 2015 Nov 26. Plant Mol Biol. 2016. PMID: 26608698
-
New perspectives on proanthocyanidin biochemistry and molecular regulation.Phytochemistry. 2003 Sep;64(2):367-83. doi: 10.1016/s0031-9422(03)00377-7. Phytochemistry. 2003. PMID: 12943753 Review.
-
The mysteries of proanthocyanidin transport and polymerization.Plant Physiol. 2010 Jun;153(2):437-43. doi: 10.1104/pp.110.155432. Epub 2010 Apr 13. Plant Physiol. 2010. PMID: 20388668 Free PMC article. Review. No abstract available.
Cited by
-
The similar and different evolutionary trends of MATE family occurred between rice and Arabidopsis thaliana.BMC Plant Biol. 2016 Sep 26;16(1):207. doi: 10.1186/s12870-016-0895-0. BMC Plant Biol. 2016. PMID: 27669820 Free PMC article.
-
QTLs Regulating the Contents of Antioxidants, Phenolics, and Flavonoids in Soybean Seeds Share a Common Genomic Region.Front Plant Sci. 2016 Jun 14;7:854. doi: 10.3389/fpls.2016.00854. eCollection 2016. Front Plant Sci. 2016. PMID: 27379137 Free PMC article.
-
Plant ABC Transporters.Arabidopsis Book. 2011;9:e0153. doi: 10.1199/tab.0153. Epub 2011 Dec 6. Arabidopsis Book. 2011. PMID: 22303277 Free PMC article.
-
Expression of the R2R3-MYB transcription factor TaMYB14 from Trifolium arvense activates proanthocyanidin biosynthesis in the legumes Trifolium repens and Medicago sativa.Plant Physiol. 2012 Jul;159(3):1204-20. doi: 10.1104/pp.112.195420. Epub 2012 May 7. Plant Physiol. 2012. PMID: 22566493 Free PMC article.
-
Behind the Scenes of Anthocyanins-From the Health Benefits to Potential Applications in Food, Pharmaceutical and Cosmetic Fields.Nutrients. 2022 Dec 2;14(23):5133. doi: 10.3390/nu14235133. Nutrients. 2022. PMID: 36501163 Free PMC article. Review.
References
-
- Abrahams, S., Lee, E., Walker, A.R., Tanner, G.J., Larkin, P.J., and Ashton, A.R. (2003). The Arabidopsis TDS4 gene encodes leucoanthocyanidin dioxygenase (LDOX) and is essential for proanthocyanidin synthesis and vacuole development. Plant J. 35 624–636. - PubMed
-
- Albert, S., Delseny, M., and Devic, M. (1997). BANYULS, a novel negative regulator of flavonoid biosynthesis in the Arabidopsis seed coat. Plant J. 11 289–299. - PubMed
-
- Baxter, I.R., Young, J.C., Armstrong, G., Foster, N., Bogenschutz, N., Cordova, T., Peer, W.A., Hazen, S.P., Murphy, A.S., and Harper, J.F. (2005). A plasma membrane H+-ATPase is required for the formation of proanthocyanidins in the seed coat endothelium of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 102 2649–2654. - PMC - PubMed
-
- Brown, M.H., Paulsen, I.T., and Skurray, R.A. (1999). The multidrug efflux protein NorM is a prototype of a new family of transporters. Mol. Microbiol. 31 394–395. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

