Capsule structural heterogeneity and antigenic variation in Cryptococcus neoformans
- PMID: 17601878
- PMCID: PMC1951120
- DOI: 10.1128/EC.00162-07
Capsule structural heterogeneity and antigenic variation in Cryptococcus neoformans
Abstract
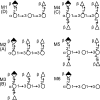
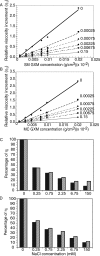
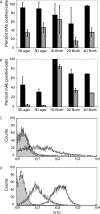
Cryptococcus neoformans is a human pathogenic fungus with a capsule composed primarily of glucuronoxylomannan (GXM) that is important for virulence. Current views of GXM structure postulate a polymer composed of repeating mannose trisaccharide motifs bearing a single beta(1,2) glucuronic acid with variable xylose and O-acetyl substitutions to form six triads. GXM from different strains is notoriously variable in triad composition, but it is not known if the polymer consists of one or more motif-repeating units. We investigated the polymeric organization of GXM by using mass spectrometry to determine if its compositional motif arrangement was similar to that of bacterial capsular polysaccharides, namely, a polymer of a single repeating unit. The results were consistent with, and confirmatory for, the current view that the basic unit of GXM is a repeating mannose trisaccharide motif, but we also found evidence for the copolymerization of different GXM repeating units in one polysaccharide molecule. Analysis of GXM from isogenic phenotypic switch variants suggested structural differences caused by glucuronic acid positional effects, which implied flexibility in the synthetic pathway. Our results suggest that cryptococcal capsule synthesis is fundamentally different from that observed in prokaryotes and employs a unique eukaryotic approach, which theoretically could synthesize an infinite number of structural combinations. The biological significance of this capsule construction scheme is that it is likely to confer a powerful avoidance strategy for interactions with the immune system and phagocytic environmental predators. Consistent with this premise, the antigenic variation of a capsular epitope recognized by a nonprotective antibody was observed under different growth conditions.
Figures



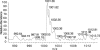

Similar articles
-
Cryptococcus neoformans Capsular GXM Conformation and Epitope Presentation: A Molecular Modelling Study.Molecules. 2020 Jun 7;25(11):2651. doi: 10.3390/molecules25112651. Molecules. 2020. PMID: 32517333 Free PMC article.
-
Structure of the O-deacetylated glucuronoxylomannan from Cryptococcus neoformans Cap70 as determined by 2D NMR spectroscopy.Carbohydr Res. 1996 Mar 22;283:95-110. doi: 10.1016/0008-6215(95)00397-5. Carbohydr Res. 1996. PMID: 8901265
-
The physical properties of the capsular polysaccharides from Cryptococcus neoformans suggest features for capsule construction.J Biol Chem. 2006 Jan 27;281(4):1868-75. doi: 10.1074/jbc.M509465200. Epub 2005 Nov 8. J Biol Chem. 2006. PMID: 16278213
-
Polysaccharides, mimotopes and vaccines for fungal and encapsulated pathogens.Trends Microbiol. 2001 Sep;9(9):445-51. doi: 10.1016/s0966-842x(01)02134-5. Trends Microbiol. 2001. PMID: 11553457 Review.
-
The capsule of the fungal pathogen Cryptococcus neoformans.Adv Appl Microbiol. 2009;68:133-216. doi: 10.1016/S0065-2164(09)01204-0. Adv Appl Microbiol. 2009. PMID: 19426855 Free PMC article. Review.
Cited by
-
Biology and function of exo-polysaccharides from human fungal pathogens.Curr Clin Microbiol Rep. 2020 Mar;7(1):1-11. doi: 10.1007/s40588-020-00137-5. Epub 2020 Jan 17. Curr Clin Microbiol Rep. 2020. PMID: 33042730 Free PMC article.
-
Polysaccharide Capsule Composition of Pneumococcal Serotype 19A Subtypes Is Unaltered among Subtypes and Independent of the Nutritional Environment.Infect Immun. 2016 Oct 17;84(11):3152-3160. doi: 10.1128/IAI.00474-16. Print 2016 Nov. Infect Immun. 2016. PMID: 27550933 Free PMC article.
-
Synthesis of a Glucuronic Acid-Containing Thioglycoside Trisaccharide Building Block and Its Use in the Assembly of Cryptococcus Neoformans Capsular Polysaccharide Fragments.ChemistryOpen. 2015 Aug 18;4(6):729-39. doi: 10.1002/open.201500143. eCollection 2015 Dec. ChemistryOpen. 2015. PMID: 27308199 Free PMC article.
-
Old Cryptococcus neoformans cells contribute to virulence in chronic cryptococcosis.mBio. 2013 Aug 13;4(4):e00455-13. doi: 10.1128/mBio.00455-13. mBio. 2013. PMID: 23943761 Free PMC article.
-
Size Matters: Measurement of Capsule Diameter in Cryptococcus neoformans.J Vis Exp. 2018 Feb 27;(132):57171. doi: 10.3791/57171. J Vis Exp. 2018. PMID: 29553511 Free PMC article.
References
-
- Casadevall, A., J. Mukherjee, S. J. Devi, R. Schneerson, J. B. Robbins, and M. D. Scharff. 1992. Antibodies elicited by a Cryptococcus neoformans glucuronoxylomannan-tetanus toxoid conjugate vaccine have the same specificity as those elicited in infection. J. Infect. Dis. 65:1086-1093. - PubMed
-
- Cherniak, R., E. Reiss, and S. Turner. 1982. A galactoxylomannan antigen of Cryptococcus neoformans serotype A. Carbohydr. Res. 103:239-250.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

