MpkA-Dependent and -independent cell wall integrity signaling in Aspergillus nidulans
- PMID: 17601879
- PMCID: PMC1951132
- DOI: 10.1128/EC.00281-06
MpkA-Dependent and -independent cell wall integrity signaling in Aspergillus nidulans
Abstract
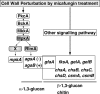
Cell wall integrity signaling (CWIS) maintains cell wall biogenesis in fungi, but only a few transcription factors (TFs) and target genes downstream of the CWIS cascade in filamentous fungi are known. Because a mitogen-activated protein kinase (MpkA) is a key CWIS enzyme, the transcriptional regulation of mpkA and of cell wall-related genes (CWGs) is important in cell wall biogenesis. We cloned Aspergillus nidulans mpkA; rlmA, a TF gene orthologous to Saccharomyces cerevisiae RLM1 that encodes Rlm1p, a major Mpk1p-dependent TF that regulates the transcription of MPK1 besides that of CWGs; and Answi4 and Answi6, homologous to S. cerevisiae SWI4 and SWI6, encoding the Mpk1p-activating TF complex Swi4p-Swi6p, which regulates CWG transcription in a cell cycle-dependent manner. A. nidulans rlmA and mpkA cDNA functionally complemented S. cerevisiae rlm1Delta and mpk1Delta mutants, respectively, but Answi4 and Answi6 cDNA did not complement swi4Delta and swi6Delta mutants. We constructed A. nidulans rlmA, Answi4 and Answi6, and mpkA disruptants (rlmADelta, Answi4Delta Answi6Delta, and mpkADelta strains) and analyzed mpkA and CWG transcripts after treatment with a beta-1,3-glucan synthase inhibitor (micafungin) that could activate MpkA via CWIS. Levels of mpkA transcripts in the mutants as well as those in the wild type were changed after micafungin treatment. The beta-glucuronidase reporter gene controlled by the mpkA promoter was expressed in the wild type but not in the mpkADelta strain. Thus, mpkA transcription seems to be autoregulated by CWIS via MpkA but not by RlmA or AnSwi4-AnSwi6. The transcription of most CWGs except alpha-1,3-glucan synthase genes (agsA and agsB) was independent of RlmA and AnSwi4-AnSwi6 and seemed to be regulated by non-MpkA signaling. The transcriptional regulation of mpkA and of CWGs via CWIS in A. nidulans differs significantly from that in S. cerevisiae.
Figures





Similar articles
-
Mitogen-activated protein kinases MpkA and MpkB independently affect micafungin sensitivity in Aspergillus nidulans.Biosci Biotechnol Biochem. 2015;79(5):836-44. doi: 10.1080/09168451.2014.998619. Epub 2015 Mar 2. Biosci Biotechnol Biochem. 2015. PMID: 25727969
-
Protein kinase C regulates the expression of cell wall-related genes in RlmA-dependent and independent manners in Aspergillus nidulans.Biosci Biotechnol Biochem. 2015;79(2):321-30. doi: 10.1080/09168451.2014.973365. Epub 2014 Oct 27. Biosci Biotechnol Biochem. 2015. PMID: 25345444
-
Effect of cell wall integrity stress and RlmA transcription factor on asexual development and autolysis in Aspergillus nidulans.Fungal Genet Biol. 2013 May;54:1-14. doi: 10.1016/j.fgb.2013.02.004. Epub 2013 Feb 26. Fungal Genet Biol. 2013. PMID: 23485399
-
Aspergillus fumigatus MADS-Box Transcription Factor rlmA Is Required for Regulation of the Cell Wall Integrity and Virulence.G3 (Bethesda). 2016 Sep 8;6(9):2983-3002. doi: 10.1534/g3.116.031112. G3 (Bethesda). 2016. PMID: 27473315 Free PMC article.
-
Cell cycle mutants.Annu Rev Genet. 1978;12:161-91. doi: 10.1146/annurev.ge.12.120178.001113. Annu Rev Genet. 1978. PMID: 106766 Review. No abstract available.
Cited by
-
Mitogen-activated protein kinases are associated with the regulation of physiological traits and virulence in Fusarium oxysporum f. sp. cubense.PLoS One. 2015 Apr 7;10(4):e0122634. doi: 10.1371/journal.pone.0122634. eCollection 2015. PLoS One. 2015. PMID: 25849862 Free PMC article.
-
Insight into the antifungal mechanism of Neosartorya fischeri antifungal protein.Protein Cell. 2015 Jul;6(7):518-28. doi: 10.1007/s13238-015-0167-z. Epub 2015 May 22. Protein Cell. 2015. PMID: 25994413 Free PMC article.
-
Velvet-mediated repression of β-glucan synthesis in Aspergillus nidulans spores.Sci Rep. 2015 May 11;5:10199. doi: 10.1038/srep10199. Sci Rep. 2015. PMID: 25960370 Free PMC article.
-
Potential Antifungal Targets for Aspergillus sp. from the Calcineurin and Heat Shock Protein Pathways.Int J Mol Sci. 2022 Oct 19;23(20):12543. doi: 10.3390/ijms232012543. Int J Mol Sci. 2022. PMID: 36293395 Free PMC article. Review.
-
Role of the guanine nucleotide exchange factor Rom2 in cell wall integrity maintenance of Aspergillus fumigatus.Eukaryot Cell. 2013 Feb;12(2):288-98. doi: 10.1128/EC.00246-12. Epub 2012 Dec 21. Eukaryot Cell. 2013. PMID: 23264643 Free PMC article.
References
-
- Aleksenko, A., I. Nikolaev, Y. Vinetski, and A. J. Clutterbuck. 1996. Gene expression from replicating plasmids in Aspergillus nidulans. Mol. Gen. Genet. 253:242-246. - PubMed
-
- Bussink, H. J., and S. A. Osmani. 1999. A mitogen-activated protein kinase (MPKA) is involved in polarized growth in the filamentous fungus, Aspergillus nidulans. FEMS Microbiol. Lett. 173:117-125. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

