Leukemia stem cells in a genetically defined murine model of blast-crisis CML
- PMID: 17601986
- PMCID: PMC1988942
- DOI: 10.1182/blood-2007-02-073031
Leukemia stem cells in a genetically defined murine model of blast-crisis CML
Abstract
Myeloid leukemia arises from leukemia stem cells (LSCs), which are resistant to standard chemotherapy agents and likely to be a major cause of drug-resistant disease and relapse. To investigate the in vivo properties of LSCs, we developed a mouse model in which the biologic features of human LSCs are closely mimicked. Primitive normal hematopoietic cells were modified to express the BCR/ABL and Nup98/HoxA9 translocation products, and a distinct LSC population, with the aberrant immunophenotype of lineage(-), Kit(+/-), Flt3(+), Sca(+), CD34(+), and CD150(-), was identified. In vivo studies were then performed to assess the response of LSCs to therapeutic insult. Treatment of animals with the ABL kinase inhibitor imatinib mesylate induced specific modulation of blasts and progenitor cells but not stem- cell populations, thereby recapitulating events inferred to occur in human chronic myelogenous leukemia (CML) patients. In addition, challenge of leukemic mice with total body irradiation was selectively toxic to normal hematopoietic stem cells (HSCs), suggesting that LSCs are resistant to apoptosis and/or senescence in vivo. Taken together, the system provides a powerful means by which the in vivo behavior of LSCs versus HSCs can be characterized and candidate treatment regimens can be optimized for maximal specificity toward primitive leukemia cells.
Figures
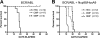



References
-
- Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. - PubMed
-
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. - PubMed
-
- Guan Y, Gerhard B, Hogge DE. Detection, isolation, and stimulation of quiescent primitive leukemic progenitor cells from patients with acute myeloid leukemia (AML). Blood. 2003;101:3142–3149. - PubMed
-
- Jordan CT. Unique molecular and cellular features of acute myelogenous leukemia stem cells. Leukemia. 2002;16:559–562. - PubMed
-
- Holyoake TL, Jiang X, Drummond MW, Eaves AC, Eaves CJ. Elucidating critical mechanisms of deregulated stem cell turnover in the chronic phase of chronic myeloid leukemia. Leukemia. 2002;16:549–558. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

