Intratumoral injection of inactivated Sendai virus particles elicits strong antitumor activity by enhancing local CXCL10 expression and systemic NK cell activation
- PMID: 17602226
- PMCID: PMC11030187
- DOI: 10.1007/s00262-007-0351-y
Intratumoral injection of inactivated Sendai virus particles elicits strong antitumor activity by enhancing local CXCL10 expression and systemic NK cell activation
Abstract
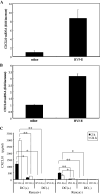
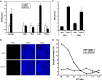
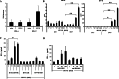
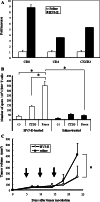
We have already demonstrated that inactivated, replication-defective Sendai virus particles (HVJ-E) have a powerful antitumor effect by both the generation of tumor-specific cytotoxic T cells and inhibition of regulatory T cell activity. Here, we report that HVJ-E also has an antitumor effect through non-T cell immunity. Microarray analysis revealed that direct injection of HVJ-E induced the expression of CXCL10 in established Renca tumors. CXCL10 was secreted by dendritic cells in the tumors after HVJ-E injection. Quantitative real-time RT-PCR and immunohistochemistry revealed that CXCR3+ cells (predominantly NK cells) infiltrated the HVJ-E-injected tumors. Moreover, HVJ-E injection caused systemic activation of NK cells and enhanced their cytotoxity against tumor cells. In an in vivo experiment, approximately 50% of tumors were eradicated by HVJ-E injection, and this activity of HVJ-E against Renca tumors was largely abolished by NK cell depletion using anti-asialo GM1 antibody. Since HVJ-E injection induced systemic antitumor immunity by enhancing or correcting the chemokine-chemokine receptor axis, it might be a potential new therapy for cancer.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

