Regulation of Nox and Duox enzymatic activity and expression
- PMID: 17602947
- PMCID: PMC1989153
- DOI: 10.1016/j.freeradbiomed.2007.03.028
Regulation of Nox and Duox enzymatic activity and expression
Abstract
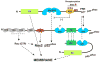
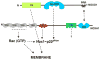
In recent years, it has become clear that reactive oxygen species (ROS, which include superoxide, hydrogen peroxide, and other metabolites) are produced in biological systems. Rather than being simply a by-product of aerobic metabolism, it is now recognized that specific enzymes--the Nox (NADPH oxidase) and Duox (Dual oxidase) enzymes--seem to have the sole function of generating ROS in a carefully regulated manner, and key roles in signal transduction, immune function, hormone biosynthesis, and other normal biological functions are being uncovered. The prototypical Nox is the respiratory burst oxidase or phagocyte oxidase, which generates large amounts of superoxide and other reactive species in the phagosomes of neutrophils and macrophages, playing a central role in innate immunity by killing microbes. This enzyme system has been extensively studied over the past two decades, and provides a basis for comparison with the more recently described Nox and Duox enzymes, which generate ROS in a variety of cells and tissues. This review first considers the structure and regulation of the respiratory burst oxidase, and then reviews recent studies relating to the regulation of the activity of the novel Nox/Duox enzymes. The regulation of Nox and Duox expression in tissues and by specific stimuli is also considered here. An accompanying review considers biological and pathological roles of the Nox family of enzymes.
Figures


References
-
- Cross A, Rae J, Curnutte J. Cytochrome b-245 of the neutrophil superoxide-generating system contains two nonidentical hemes. Journal of Biological Chemistry. 1995;270:17075–17077. - PubMed
-
- Bibersine-Kinkade KJ, DeLeo FR, Epstein RI, LeRoy BA, Bauseef WM, Dinauer MC. Heme-ligating histidines in flavocytochrome b558. Identification of specific histidines in gp91phox. Journal of Biological Chemistry. 2001;276:31105–31112. - PubMed
-
- Mankelow TJ, Henderson LM. Proton conduction through full-length gp91phox requires histidine 115. Protoplasma. 2003;221:101–108. - PubMed
-
- Isogai Y, Iizuka T, Shiro Y. The mechanism of electron donation to molecular oxygen by phagocytic cytochrome b558. J Biol Chem. 1995;270:7853–7857. - PubMed
-
- Biberstine-Kinkade B, Yu L, Dinauer M. Mutagenesis of an arginine- and lysine-rich domain in the gp91phox subunit of the phagocyte NADPH-oxidase flavocytochrome b558. Journal of Biological Chemistry. 1999;274:10451–10457. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

