De novo synthesis of glutathione is a prerequisite for curcumin to inhibit hepatic stellate cell (HSC) activation
- PMID: 17602960
- PMCID: PMC2562715
- DOI: 10.1016/j.freeradbiomed.2007.04.016
De novo synthesis of glutathione is a prerequisite for curcumin to inhibit hepatic stellate cell (HSC) activation
Abstract
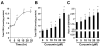
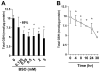
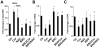
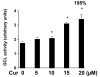
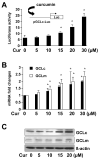
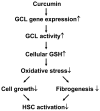
On liver injury, quiescent hepatic stellate cells (HSC), the most relevant cell type for hepatic fibrogenesis, become active, characterized by enhanced cell growth and overproduction of extracellular matrix (ECM). Oxidative stress facilitates HSC activation and the pathogenesis of hepatic fibrosis. Glutathione (GSH) is the most important intracellular antioxidant. We previously showed that curcumin, the yellow pigment in curry from turmeric, significantly inhibited HSC activation. The aim of this study is to elucidate the underlying mechanisms. It is hypothesized that curcumin might inhibit HSC activation mainly by its antioxidant capacity. Results from this study demonstrate that curcumin dose and time dependently attenuates oxidative stress in passaged HSC demonstrated by scavenging reactive oxygen species and reducing lipid peroxidation. Curcumin elevates the level of cellular GSH and induces de novo synthesis of GSH in HSC by stimulating the activity and gene expression of glutamate-cysteine ligase (GCL), a key rate-limiting enzyme in GSH synthesis. Depletion of cellular GSH by the inhibition of GCL activity using L-buthionine sulfoximine evidently eliminates the inhibitory effects of curcumin on HSC activation. Taken together, our results demonstrate, for the first time, that the antioxidant property of curcumin mainly results from increasing the level of cellular GSH by inducing the activity and gene expression of GCL in activated HSC in vitro. De novo synthesis of GSH is a prerequisite for curcumin to inhibit HSC activation. These results provide novel insights into the mechanisms of curcumin as an antifibrogenic candidate in the prevention and treatment of hepatic fibrosis.
Figures



Similar articles
-
The antifibrogenic effect of (-)-epigallocatechin gallate results from the induction of de novo synthesis of glutathione in passaged rat hepatic stellate cells.Lab Invest. 2006 Jul;86(7):697-709. doi: 10.1038/labinvest.3700425. Epub 2006 May 8. Lab Invest. 2006. PMID: 16682975
-
Curcumin attenuates the effects of insulin on stimulating hepatic stellate cell activation by interrupting insulin signaling and attenuating oxidative stress.Lab Invest. 2009 Dec;89(12):1397-409. doi: 10.1038/labinvest.2009.115. Epub 2009 Oct 19. Lab Invest. 2009. PMID: 19841616 Free PMC article.
-
Epigallocatechin-3-gallate inhibits growth of activated hepatic stellate cells by enhancing the capacity of glutathione synthesis.Mol Pharmacol. 2008 May;73(5):1465-73. doi: 10.1124/mol.107.040634. Epub 2008 Jan 29. Mol Pharmacol. 2008. PMID: 18230716 Free PMC article.
-
Cooperation of liver cells in health and disease.Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review.
-
Glutathione defense mechanism in liver injury: insights from animal models.Food Chem Toxicol. 2013 Oct;60:38-44. doi: 10.1016/j.fct.2013.07.008. Epub 2013 Jul 12. Food Chem Toxicol. 2013. PMID: 23856494 Free PMC article. Review.
Cited by
-
Curcumin: potential for hepatic fibrosis therapy?Br J Pharmacol. 2008 Feb;153(3):403-5. doi: 10.1038/sj.bjp.0707580. Epub 2007 Nov 26. Br J Pharmacol. 2008. PMID: 18037917 Free PMC article.
-
Mitochondria-targeted curcumin loaded CTPP-PEG-PCL self-assembled micelles for improving liver fibrosis therapy.RSC Adv. 2021 Jan 28;11(10):5348-5360. doi: 10.1039/d0ra09589c. eCollection 2021 Jan 28. RSC Adv. 2021. PMID: 35423083 Free PMC article.
-
Perilipin 5 restores the formation of lipid droplets in activated hepatic stellate cells and inhibits their activation.Lab Invest. 2016 Jul;96(7):791-806. doi: 10.1038/labinvest.2016.53. Epub 2016 May 2. Lab Invest. 2016. PMID: 27135793
-
Curcumin and vitamin E modulate hepatic antioxidant gene expression in PTU-induced hypothyroid rats.Mol Biol Rep. 2012 Nov;39(11):9849-61. doi: 10.1007/s11033-012-1851-1. Epub 2012 Jun 24. Mol Biol Rep. 2012. PMID: 22733496
-
Expression of antioxidant genes in renal cortex of PTU-induced hypothyroid rats: effect of vitamin E and curcumin.Mol Biol Rep. 2012 Feb;39(2):1193-203. doi: 10.1007/s11033-011-0849-4. Epub 2011 May 24. Mol Biol Rep. 2012. PMID: 21607622
References
-
- Bissell DM. Hepatic fibrosis as wound repair: a progress report. J Gastroenterol. 1998;33:295–302. - PubMed
-
- Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38(Suppl 1):S38–53. - PubMed
-
- Kisseleva T, Brenner DA. Hepatic stellate cells and the reversal of fibrosis. J Gastroenterol Hepatol. 2006;21(Suppl 3):S84–87. - PubMed
-
- Fernandez-Checa JC, Kaplowitz N, Garcia-Ruiz C, Colell A. Mitochondrial glutathione: importance and transport. Semin Liver Dis. 1998;18:389–401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

