Low dose combination of morphine and delta9-tetrahydrocannabinol circumvents antinociceptive tolerance and apparent desensitization of receptors
- PMID: 17603035
- PMCID: PMC2040345
- DOI: 10.1016/j.ejphar.2007.06.001
Low dose combination of morphine and delta9-tetrahydrocannabinol circumvents antinociceptive tolerance and apparent desensitization of receptors
Abstract
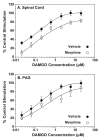
Morphine and delta9-tetrahydrocannabinol (THC) produce antinociception via mu opioid and cannabinoid CB1 receptors, respectively, located in central nervous system (CNS) regions including periaqueductal gray and spinal cord. Chronic treatment with morphine or THC produces antinociceptive tolerance and cellular adaptations that include receptor desensitization. Previous studies have shown that administration of combined sub-analgesic doses of THC+morphine produced antinociception in the absence of tolerance. The present study assessed receptor-mediated G-protein activity in spinal cord and periaqueductal gray following chronic administration of THC, morphine or low dose combination. Rats received morphine (escalating doses from 1 to 6x75 mg s.c. pellets or s.c. injection of 100 to 200 mg/kg twice daily), THC (4 mg/kg i.p. twice daily) or low dose combination (0.75 mg/kg each morphine (s.c) and THC (i.p.) twice daily) for 6.5 days. Antinociception was measured in one cohort of rats using the paw pressure test, and a second cohort was assessed for agonist-stimulated [35S]GTPgammaS binding. Chronic administration of morphine or THC produced antinociceptive tolerance to the respective drugs, whereas combination treatment did not produce tolerance. Administration of THC attenuated cannabinoid CB1 receptor-stimulated G-protein activity in both periaqueductal gray and spinal cord, and administration of morphine decreased mu opioid receptor-stimulated [35S]GTPgammaS binding in spinal cord or periaqueductal gray, depending on route of administration. In contrast, combination treatment did not alter cannabinoid CB1 receptor- or mu opioid receptor-stimulated G-protein activity in either region. These results demonstrate that low dose THC-morphine combination treatment produces antinociception in the absence of tolerance or attenuation of receptor activity.
Figures





Similar articles
-
The periaqueductal gray contributes to bidirectional enhancement of antinociception between morphine and cannabinoids.Pharmacol Biochem Behav. 2013 Jan;103(3):444-9. doi: 10.1016/j.pbb.2012.10.002. Epub 2012 Oct 10. Pharmacol Biochem Behav. 2013. PMID: 23063785 Free PMC article.
-
Time-dependent differences of repeated administration with Delta9-tetrahydrocannabinol in proenkephalin and cannabinoid receptor gene expression and G-protein activation by mu-opioid and CB1-cannabinoid receptors in the caudate-putamen.Brain Res Mol Brain Res. 1999 Apr 6;67(1):148-57. doi: 10.1016/s0169-328x(99)00053-4. Brain Res Mol Brain Res. 1999. PMID: 10101241
-
[(35)S]GTPγS binding and opioid tolerance and efficacy in mouse spinal cord.Pharmacol Biochem Behav. 2012 Mar;101(1):155-65. doi: 10.1016/j.pbb.2011.11.001. Epub 2011 Nov 12. Pharmacol Biochem Behav. 2012. PMID: 22108651
-
Cannabinoid-opioid interactions during neuropathic pain and analgesia.Curr Opin Pharmacol. 2010 Feb;10(1):80-6. doi: 10.1016/j.coph.2009.09.009. Epub 2009 Oct 25. Curr Opin Pharmacol. 2010. PMID: 19857996 Free PMC article. Review.
-
Preclinical pharmacology and opioid combinations.Pain Med. 2012 Mar;13 Suppl 1(s1):S4-11. doi: 10.1111/j.1526-4637.2012.01335.x. Pain Med. 2012. PMID: 22420604 Free PMC article. Review.
Cited by
-
Positive Allosteric Modulation of CB1 Cannabinoid Receptor Signaling Enhances Morphine Antinociception and Attenuates Morphine Tolerance Without Enhancing Morphine- Induced Dependence or Reward.Front Mol Neurosci. 2020 Apr 28;13:54. doi: 10.3389/fnmol.2020.00054. eCollection 2020. Front Mol Neurosci. 2020. PMID: 32410959 Free PMC article.
-
[Cannabinoids in pain medicine].Schmerz. 2018 Oct;32(5):381-396. doi: 10.1007/s00482-018-0299-1. Schmerz. 2018. PMID: 29881935 German.
-
In vivo characterization of MMP-2200, a mixed δ/μ opioid agonist, in mice.J Pharmacol Exp Ther. 2011 Mar;336(3):767-78. doi: 10.1124/jpet.110.172866. Epub 2010 Nov 30. J Pharmacol Exp Ther. 2011. PMID: 21118955 Free PMC article.
-
Characterization of cannabinoid-1 receptors in the locus coeruleus: relationship with mu-opioid receptors.Brain Res. 2010 Feb 2;1312:18-31. doi: 10.1016/j.brainres.2009.11.023. Epub 2009 Nov 18. Brain Res. 2010. PMID: 19931229 Free PMC article.
-
Emerging Evidence for Cannabis' Role in Opioid Use Disorder.Cannabis Cannabinoid Res. 2018 Sep 1;3(1):179-189. doi: 10.1089/can.2018.0022. eCollection 2018. Cannabis Cannabinoid Res. 2018. PMID: 30221197 Free PMC article. Review.
References
-
- Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Ann Rev Neurosci. 1984;7:309–338. - PubMed
-
- Bloom AS, Dewey WL. A comparison of some pharmacological actions of morphine and delta9-tetrahydrocannabinol in the mouse. Psychopharmacology (Berl) 1978;57:243–248. - PubMed
-
- Childers SR. Opioid receptor-coupled second messengers. Life Sci. 1991;48:1991–2003. - PubMed
-
- Cichewicz DL. Synergistic interactions between cannabinoid and opioid analgesics. Life Sci. 2004;74:1317–1324. - PubMed
-
- Cichewicz DL, Martin ZL, Smith FL, Welch SP. Enhancement mu opioid antinociception by oral delta9-tetrahydrocannabinol: dose-response analysis and receptor identification. J Pharmacol Exp Ther. 1999;289:859–867. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

