Homeostatic mechanisms for iron storage revealed by genetic manipulations and live imaging of Drosophila ferritin
- PMID: 17603097
- PMCID: PMC2013694
- DOI: 10.1534/genetics.107.075150
Homeostatic mechanisms for iron storage revealed by genetic manipulations and live imaging of Drosophila ferritin
Abstract
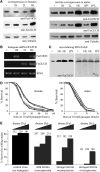
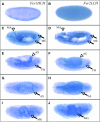
Ferritin is a symmetric, 24-subunit iron-storage complex assembled of H and L chains. It is found in bacteria, plants, and animals and in two classes of mutations in the human L-chain gene, resulting in hereditary hyperferritinemia cataract syndrome or in neuroferritinopathy. Here, we examined systemic and cellular ferritin regulation and trafficking in the model organism Drosophila melanogaster. We showed that ferritin H and L transcripts are coexpressed during embryogenesis and that both subunits are essential for embryonic development. Ferritin overexpression impaired the survival of iron-deprived flies. In vivo expression of GFP-tagged holoferritin confirmed that iron-loaded ferritin molecules traffic through the Golgi organelle and are secreted into hemolymph. A constant ratio of ferritin H and L subunits, secured via tight post-transcriptional regulation, is characteristic of the secreted ferritin in flies. Differential cellular expression, conserved post-transcriptional regulation via the iron regulatory element, and distinct subcellular localization of the ferritin subunits prior to the assembly of holoferritin are all important steps mediating iron homeostasis. Our study revealed both conserved features and insect-specific adaptations of ferritin nanocages and provides novel imaging possibilities for their in vivo characterization.
Figures




References
-
- Bauer, M., J. D. Katzenberger, A. C. Hamm, M. Bonaus, I. Zinke et al., 2006. Purine and folate metabolism as a potential target of sex-specific nutrient allocation in Drosophila and its implication for lifespan-reproduction tradeoff. Physiol. Genomics 25: 393–404. - PubMed
-
- Beutler, E., V. Felitti, N. J. Ho and T. Gelbart, 2002. Relationship of body iron stores to levels of serum ferritin, serum iron, unsaturated iron binding capacity and transferrin saturation in patients with iron storage disease. Acta Haematol. 107: 145–149. - PubMed
-
- Brand, A. H., and N. Perrimon, 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415. - PubMed
-
- Brody, T., C. Stivers, J. Nagle and W. F. Odenwald, 2002. Identification of novel Drosophila neural precursor genes using a differential embryonic head cDNA screen. Mech. Dev. 113: 41–59. - PubMed
-
- Capurro Mde, L., P. Iughetti, P. E. Ribolla and A. G. de Bianchi, 1996. Musca domestica hemolymph ferritin. Arch. Insect Biochem. Physiol. 32: 197–207. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

