Human UGT1A8 and UGT1A10 mRNA are expressed in primary human hepatocytes
- PMID: 17603215
- PMCID: PMC2275121
- DOI: 10.2133/dmpk.22.152
Human UGT1A8 and UGT1A10 mRNA are expressed in primary human hepatocytes
Abstract
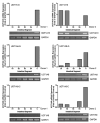
It is widely believed that the UGT1A isoforms, UGT1A8 and -1A10, are expressed exclusively in extrahepatic tissues. In this work, human primary hepatocytes from six donors were analyzed for UGT1A8 and -1A10 mRNA expression by semi-quantitative RT-PCR. New primers to amplify UGT1A8 mRNA were designed and found to differ from those previously published. We demonstrated that UGT1A8 and -1A10 mRNA are expressed in hepatocytes. Although basal UGT mRNA levels were detected in untreated hepatocytes, significant up-regulation of the levels of mRNA for these isoforms were seen after treatment with 3-methylcholanthrene (3-MC) and rifampicin (Rif). RT-PCR products for all UGTs were sequenced and unambiguously identified as matching the corresponding cDNA. The discovery of these isoforms in hepatocytes is a novel discovery and will stimulate studies on the potential role for these isoforms in hepatic detoxification.
Figures


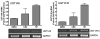


Similar articles
-
Coordinate regulation of the human UDP-glucuronosyltransferase 1A8, 1A9, and 1A10 genes by hepatocyte nuclear factor 1alpha and the caudal-related homeodomain protein 2.Mol Pharmacol. 2004 Apr;65(4):953-63. doi: 10.1124/mol.65.4.953. Mol Pharmacol. 2004. PMID: 15044625
-
Polymorphic expression of the UDP-glucuronosyltransferase UGT1A gene locus in human gastric epithelium.Mol Pharmacol. 1998 Oct;54(4):647-54. Mol Pharmacol. 1998. PMID: 9765507
-
Expression of the UDP-glucuronosyltransferase 1A locus in human colon. Identification and characterization of the novel extrahepatic UGT1A8.J Biol Chem. 1998 Apr 10;273(15):8719-26. doi: 10.1074/jbc.273.15.8719. J Biol Chem. 1998. PMID: 9535849
-
Regulation of UDP glucuronosyltransferases in the gastrointestinal tract.Toxicol Appl Pharmacol. 2004 Sep 15;199(3):354-63. doi: 10.1016/j.taap.2004.01.008. Toxicol Appl Pharmacol. 2004. PMID: 15364550 Review.
-
18β-glycyrrhetinic acid induces UDP-glucuronosyltransferase in rats.Protein Pept Lett. 2013 Dec;20(12):1360-4. doi: 10.2174/092986652012131112124033. Protein Pept Lett. 2013. PMID: 24261979 Review.
Cited by
-
Quantification of human uridine-diphosphate glucuronosyl transferase 1A isoforms in liver, intestine, and kidney using nanobore liquid chromatography-tandem mass spectrometry.Anal Chem. 2012 Jan 3;84(1):98-105. doi: 10.1021/ac201704a. Epub 2011 Dec 5. Anal Chem. 2012. PMID: 22050083 Free PMC article.
-
Pharmacogenomics: a new paradigm to personalize treatments in nephrology patients.Clin Exp Immunol. 2010 Mar;159(3):268-80. doi: 10.1111/j.1365-2249.2009.04065.x. Epub 2009 Nov 24. Clin Exp Immunol. 2010. PMID: 19968662 Free PMC article. Review.
-
Tissue-specific and ubiquitous expression patterns from alternative promoters of human genes.PLoS One. 2010 Aug 18;5(8):e12274. doi: 10.1371/journal.pone.0012274. PLoS One. 2010. PMID: 20806066 Free PMC article.
-
Dopamine is a low-affinity and high-specificity substrate for the human UDP-glucuronosyltransferase 1A10.Drug Metab Dispos. 2009 Apr;37(4):768-75. doi: 10.1124/dmd.108.025692. Epub 2008 Dec 30. Drug Metab Dispos. 2009. PMID: 19116261 Free PMC article.
-
Disposition of Oral Nalbuphine and Its Metabolites in Healthy Subjects and Subjects with Hepatic Impairment: Preliminary Modeling Results Using a Continuous Intestinal Absorption Model with Enterohepatic Recirculation.Metabolites. 2024 Aug 27;14(9):471. doi: 10.3390/metabo14090471. Metabolites. 2024. PMID: 39330478 Free PMC article.
References
-
- Dutton GJ. Glucuronidation of Drugs and Other Compounds. CRC Press Inc.; Boca Raton, Fl: 1980.
-
- Ritter JK, Chen F, Sheen YY, Tran HM, Kimura S, Yeatman MT, Owens IS. A novel complex locus UGT1 encodes human bilirubin, phenol, and other UDP-glucuronosyltransferase isozymes with identical carboxyl termini. J Biol Chem. 1992;267:3257–3261. - PubMed
-
- Mackenzie PI. The UDP-glucuronosyltransferase multigene family. Toxicology Communications Inc.; Raleigh: 1995. pp. 29–72.
-
- Mackenzie PI, Walter Bock K, Burchell B, Guillemette C, Ikushiro S, Iyanagi T, Miners JO, Owens IS, Nebert DW. Nomenclature update for the mammalian UDP glycosyltransferase (UGT) gene superfamily. Pharmacogenet Genomics. 2005;15:677–685. - PubMed
-
- Ritter JK, Crawford JM, Owens IS. Cloning of two human liver bilirubin UDP-glucuronosyltransferase cDNAs with expression in COS-1 cells. J Biol Chem. 1991;266:1043–1047. - PubMed
