Structure-based activity prediction for an enzyme of unknown function
- PMID: 17603473
- PMCID: PMC2254328
- DOI: 10.1038/nature05981
Structure-based activity prediction for an enzyme of unknown function
Abstract
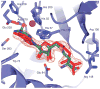
With many genomes sequenced, a pressing challenge in biology is predicting the function of the proteins that the genes encode. When proteins are unrelated to others of known activity, bioinformatics inference for function becomes problematic. It would thus be useful to interrogate protein structures for function directly. Here, we predict the function of an enzyme of unknown activity, Tm0936 from Thermotoga maritima, by docking high-energy intermediate forms of thousands of candidate metabolites. The docking hit list was dominated by adenine analogues, which appeared to undergo C6-deamination. Four of these, including 5-methylthioadenosine and S-adenosylhomocysteine (SAH), were tested as substrates, and three had substantial catalytic rate constants (10(5) M(-1 )s(-1)). The X-ray crystal structure of the complex between Tm0936 and the product resulting from the deamination of SAH, S-inosylhomocysteine, was determined, and it corresponded closely to the predicted structure. The deaminated products can be further metabolized by T. maritima in a previously uncharacterized SAH degradation pathway. Structure-based docking with high-energy forms of potential substrates may be a useful tool to annotate enzymes for function.
Figures

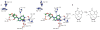
Comment in
-
Computational biochemistry: models of transition.Nature. 2007 Aug 16;448(7155):762-3. doi: 10.1038/448762a. Nature. 2007. PMID: 17700688 No abstract available.
References
-
- Whisstock JC, Lesk AM. Prediction of protein function from protein sequence and structure. Q Rev Biophys. 2003;36:307–340. - PubMed
-
- Brenner SE. Errors in genome annotation. Trends Genet. 1999;15:132–133. - PubMed
-
- Devos D, Valencia A. Intrinsic errors in genome annotation. Trends Genet. 2001;17:429–431. - PubMed
-
- Schapira M, Abagyan R, Totrov M. Nuclear hormone receptor targeted virtual screening. J Med Chem. 2003;46:3045–3059. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

