Mast cell lineage diversion of T lineage precursors by the essential T cell transcription factor GATA-3
- PMID: 17603486
- PMCID: PMC3140173
- DOI: 10.1038/ni1486
Mast cell lineage diversion of T lineage precursors by the essential T cell transcription factor GATA-3
Abstract
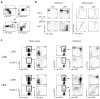
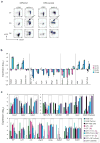
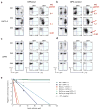
GATA-3 is essential for T cell development from the earliest stages. However, abundant GATA-3 can drive T lineage precursors to a non-T cell fate, depending on Notch signaling and developmental stage. Here, overexpression of GATA-3 blocked the survival of pro-T cells when Notch-Delta signals were present but enhanced viability in their absence. In fetal thymocytes at the double-negative 1 (DN1) stage and DN2 stage but not those at the DN3 stage, overexpression of GATA-3 rapidly induced respecification to the mast cell lineage with high frequency by direct transcriptional 'reprogramming'. Normal DN2 thymocytes also showed mast cell potential when interleukin 3 and stem cell factor were added in the absence of Notch signaling. Our results suggest a close relationship between the pro-T cell and mast cell programs and a previously unknown function for Notch in T lineage fidelity.
Figures


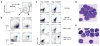
Comment in
-
No DL1 Notch ligand? GATA be a mast cell.Nat Immunol. 2007 Aug;8(8):796-8. doi: 10.1038/ni0807-796. Nat Immunol. 2007. PMID: 17641659 No abstract available.
Similar articles
-
No DL1 Notch ligand? GATA be a mast cell.Nat Immunol. 2007 Aug;8(8):796-8. doi: 10.1038/ni0807-796. Nat Immunol. 2007. PMID: 17641659 No abstract available.
-
GATA-3 dose-dependent checkpoints in early T cell commitment.J Immunol. 2014 Oct 1;193(7):3470-91. doi: 10.4049/jimmunol.1301663. Epub 2014 Aug 29. J Immunol. 2014. PMID: 25172496 Free PMC article.
-
Distinct functions for the transcription factors GATA-3 and ThPOK during intrathymic differentiation of CD4(+) T cells.Nat Immunol. 2008 Oct;9(10):1122-30. doi: 10.1038/ni.1647. Epub 2008 Sep 7. Nat Immunol. 2008. PMID: 18776904 Free PMC article.
-
Competition and collaboration: GATA-3, PU.1, and Notch signaling in early T-cell fate determination.Semin Immunol. 2008 Aug;20(4):236-46. doi: 10.1016/j.smim.2008.07.006. Epub 2008 Sep 3. Semin Immunol. 2008. PMID: 18768329 Free PMC article. Review.
-
From the cradle to the grave: activities of GATA-3 throughout T-cell development and differentiation.Immunol Rev. 2010 Nov;238(1):110-25. doi: 10.1111/j.1600-065X.2010.00954.x. Immunol Rev. 2010. PMID: 20969588 Free PMC article. Review.
Cited by
-
Transcriptional network dynamics in early T cell development.J Exp Med. 2024 Oct 7;221(10):e20230893. doi: 10.1084/jem.20230893. Epub 2024 Aug 21. J Exp Med. 2024. PMID: 39167073 Free PMC article. Review.
-
A far downstream enhancer for murine Bcl11b controls its T-cell specific expression.Blood. 2013 Aug 8;122(6):902-11. doi: 10.1182/blood-2012-08-447839. Epub 2013 Jun 5. Blood. 2013. PMID: 23741008 Free PMC article.
-
Redefinition of the human mast cell transcriptome by deep-CAGE sequencing.Blood. 2014 Apr 24;123(17):e58-67. doi: 10.1182/blood-2013-02-483792. Epub 2014 Mar 26. Blood. 2014. PMID: 24671954 Free PMC article.
-
HEB in the spotlight: Transcriptional regulation of T-cell specification, commitment, and developmental plasticity.Clin Dev Immunol. 2012;2012:678705. doi: 10.1155/2012/678705. Epub 2012 Apr 22. Clin Dev Immunol. 2012. PMID: 22577461 Free PMC article. Review.
-
Anaphylaxis: Focus on Transcription Factor Activity.Int J Mol Sci. 2021 May 6;22(9):4935. doi: 10.3390/ijms22094935. Int J Mol Sci. 2021. PMID: 34066544 Free PMC article. Review.
References
-
- Samson SI, et al. GATA-3 promotes maturation, IFN-γ production, and liver-specific homing of NK cells. Immunity. 2003;19:701–711. - PubMed
-
- Hendriks RW, et al. Expression of the transcription factor GATA-3 is required for the development of the earliest T cell progenitors and correlates with stages of cellular proliferation in the thymus. Eur J Immunol. 1999;29:1912–1918. - PubMed
-
- Ting CN, Olson MC, Barton KP, Leiden JM. Transcription factor GATA-3 is required for development of the T-cell lineage. Nature. 1996;384:474–478. - PubMed
-
- Rothenberg EV, Taghon T. Molecular Genetics of T Cell Development. Annu Rev Immunol. 2005;23:601–649. - PubMed
-
- Zhu J, Yamane H, Cote-Sierra J, Guo L, Paul WE. GATA-3 promotes Th2 responses through three different mechanisms: induction of Th2 cytokine production, selective growth of Th2 cells and inhibition of Th1 cell-specific factors. Cell Res. 2006;16:3–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

