The potential of high-content high-throughput microscopy in drug discovery
- PMID: 17603554
- PMCID: PMC1978277
- DOI: 10.1038/sj.bjp.0707346
The potential of high-content high-throughput microscopy in drug discovery
Abstract
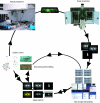
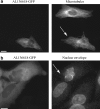
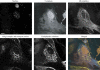
Fluorescence microscopy is a powerful method to study protein function in its natural habitat, the living cell. With the availability of the green fluorescent protein and its spectral variants, almost any gene of interest can be fluorescently labelled in living cells opening the possibility to study protein localization, dynamics and interactions. The emergence of automated cellular systems allows rapid visualization of large groups of cells and phenotypic analysis in a quantitative manner. Here, we discuss recent advances in high-content high-throughput microscopy and its potential application to several steps of the drug discovery process.
Figures
Comment in
-
High-throughput microscopy must re-invent the microscope rather than speed up its functions.Br J Pharmacol. 2007 Sep;152(1):1-4. doi: 10.1038/sj.bjp.0707348. Epub 2007 Jul 2. Br J Pharmacol. 2007. PMID: 17603553 Free PMC article.
Similar articles
-
High-throughput microscopy must re-invent the microscope rather than speed up its functions.Br J Pharmacol. 2007 Sep;152(1):1-4. doi: 10.1038/sj.bjp.0707348. Epub 2007 Jul 2. Br J Pharmacol. 2007. PMID: 17603553 Free PMC article.
-
Multidimensional drug profiling by automated microscopy.Science. 2004 Nov 12;306(5699):1194-8. doi: 10.1126/science.1100709. Science. 2004. PMID: 15539606
-
Integrated expressional analysis: application to the drug discovery process.Methods. 2005 Nov;37(3):280-8. doi: 10.1016/j.ymeth.2005.03.013. Methods. 2005. PMID: 16308157
-
Image-based screening: a technology in transition.Curr Opin Biotechnol. 2005 Feb;16(1):41-8. doi: 10.1016/j.copbio.2004.12.005. Curr Opin Biotechnol. 2005. PMID: 15722014 Review.
-
Image-based chemical screening.Nat Chem Biol. 2007 Aug;3(8):461-5. doi: 10.1038/nchembio.2007.15. Nat Chem Biol. 2007. PMID: 17637778 Review.
Cited by
-
Large population cell characterization using quantitative phase cytometer.Cytometry A. 2017 May;91(5):450-459. doi: 10.1002/cyto.a.23106. Epub 2017 Apr 26. Cytometry A. 2017. PMID: 28444998 Free PMC article.
-
Microplate-compatible total internal reflection fluorescence microscopy for receptor pharmacology.Appl Phys Lett. 2013 May 13;102(19):193702. doi: 10.1063/1.4805041. Epub 2013 May 14. Appl Phys Lett. 2013. PMID: 23825800 Free PMC article.
-
Automated analysis of time-lapse imaging of nuclear translocation by retrospective strategy and its application to STAT1 in HeLa cells.PLoS One. 2011;6(11):e27454. doi: 10.1371/journal.pone.0027454. Epub 2011 Nov 18. PLoS One. 2011. PMID: 22125613 Free PMC article.
-
Generalising from conventional pipelines using deep learning in high-throughput screening workflows.Sci Rep. 2022 Jul 6;12(1):11465. doi: 10.1038/s41598-022-15623-7. Sci Rep. 2022. PMID: 35794231 Free PMC article.
-
Total internal reflection fluorescence quantification of receptor pharmacology.Biosensors (Basel). 2015 Apr 27;5(2):223-40. doi: 10.3390/bios5020223. Biosensors (Basel). 2015. PMID: 25922915 Free PMC article. Review.
References
-
- Abraham VC, Taylor DL, Haskins JR. High content screening applied to large-scale cell biology. Trends Biotech. 2004;22:15–22. - PubMed
-
- Adams M, Celniker S, Holt R, Evans Ch, Gocayne JD, Amanatides PG, et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. - PubMed
-
- Arlt D, Huber W, Liebel U, Schmidt C, Majety M, Sauermann M, et al. Functional profiling: from microarrays via cell-based assays to novel tumor relevant modulators of the cell cycle. Cancer Res. 2005;65:7733–7742. - PubMed
-
- Bailey SN, Ali SM, Carpenter AE, Higgins CO, Sabatini DM. Microarrays of lentiviruses for gene function screens in immortalized and primary cells. Nat Methods. 2006;3:117–122. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

