Single fluorescent protein-based Ca2+ sensors with increased dynamic range
- PMID: 17603870
- PMCID: PMC1931437
- DOI: 10.1186/1472-6750-7-37
Single fluorescent protein-based Ca2+ sensors with increased dynamic range
Abstract
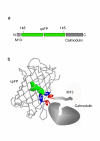
Background: Genetically encoded sensors developed on the basis of green fluorescent protein (GFP)-like proteins are becoming more and more popular instruments for monitoring cellular analytes and enzyme activities in living cells and transgenic organisms. In particular, a number of Ca2+ sensors have been developed, either based on FRET (Fluorescence Resonance Energy Transfer) changes between two GFP-mutants or on the change in fluorescence intensity of a single circularly permuted fluorescent protein (cpFP).
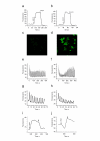
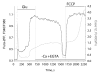
Results: Here we report significant progress on the development of the latter type of Ca2+ sensors. Derived from the knowledge of previously reported cpFP-based sensors, we generated a set of cpFP-based indicators with different spectral properties and fluorescent responses to changes in Ca2+ concentration. Two variants, named Case12 and Case16, were characterized by particular high brightness and superior dynamic range, up to 12-fold and 16.5-fold increase in green fluorescence between Ca2+-free and Ca2+-saturated forms. We demonstrated the high potential of these sensors on various examples, including monitoring of Ca2+ response to a prolonged glutamate treatment in cortical neurons.
Conclusion: We believe that expanded dynamic range, high brightness and relatively high pH-stability should make Case12 and Case16 popular research tools both in scientific studies and high throughput screening assays.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

