NQO1-activated phenothiazinium redox cyclers for the targeted bioreductive induction of cancer cell apoptosis
- PMID: 17603928
- PMCID: PMC2705808
- DOI: 10.1016/j.freeradbiomed.2007.03.035
NQO1-activated phenothiazinium redox cyclers for the targeted bioreductive induction of cancer cell apoptosis
Abstract
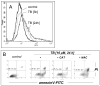
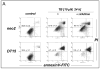
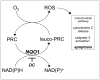
Altered redox signaling and regulation in cancer cells represent a chemical vulnerability that can be targeted by selective chemotherapeutic intervention. Here, we demonstrate that 3,7-diaminophenothiazinium-based redox cyclers (PRC) induce selective cancer cell apoptosis by NAD(P)H:quinone oxidoreductase (NQO1)-dependent bioreductive generation of cellular oxidative stress. Using PRC lead compounds including toluidine blue against human metastatic G361 melanoma cells, apoptosis occurred with phosphatidylserine externalization, loss of mitochondrial transmembrane potential, cytochrome c release, caspase-3 activation, and massive ROS production. Consistent with reductive activation and subsequent redox cycling as the mechanism of PRC cytotoxicity, coincubation with catalase achieved cell protection, whereas reductive antioxidants enhanced PRC cytotoxicity. Unexpectedly, human A375 melanoma cells were resistant to PRC-induced apoptosis, and PRC-sensitive G361 cells were protected by preincubation with the NQO1 inhibitor dicoumarol. Indeed, NQO1 specific enzymatic activity was 9-fold higher in G361 than in A375 cells. The critical role of NQO1 in PRC bioactivation and cytotoxicity was confirmed, when NQO1-transfected breast cancer cells (MCF7-DT15) stably overexpressing active NQO1 displayed strongly enhanced PRC sensitivity as compared to vector control-transfected cells with baseline NQO1 activity. Based on the known overexpression of NQO1 in various tumors these findings suggest the feasibility of developing PRC lead compounds into tumor-selective bioreductive chemotherapeutics.
Figures









References
-
- Laurent A, Nicco C, Chereau C, Goulvestre C, Alexandre J, Alves A, et al. Controlling tumor growth by modulating endogenous production of reactive oxygen species. Cancer Res. 2005;65:948–56. - PubMed
-
- Giles GI. The redox regulation of thiol dependent signaling pathways in cancer. Curr Pharm Des. 2006;12:4427–43. - PubMed
-
- Wu WS. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 2006;25:695–705. - PubMed
-
- Nishigori C, Hattori Y, Toyokuni S. Role of reactive oxygen species in skin carcinogenesis. Antioxid Redox Signal. 2004;6:561–70. - PubMed
-
- Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, et al. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

