Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V)
- PMID: 17604108
- PMCID: PMC6116895
- DOI: 10.1016/j.brainresrev.2007.05.006
Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V)
Abstract
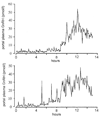
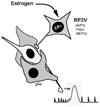
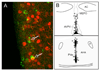
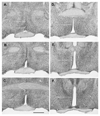
Increasing levels of circulating estradiol during the follicular phase of the ovarian cycle act on the brain to trigger a sudden and massive release of gonadotropin-releasing hormone (GnRH) that evokes the pituitary luteinizing hormone surge responsible for ovulation in mammals. The mechanisms through which estrogen is able to exert this potent "positive feedback" influence upon the GnRH neurons are beginning to be unravelled. Recent studies utilizing mouse models with global and cell-specific deletions of the different estrogen receptors (ERs) have shown that estrogen positive feedback is likely to use an indirect pathway involving the modulation of ERalpha-expressing neurons that project to GnRH neurons. Conventional tract tracing studies in rats, and experiments involving conditional pseudorabies virus tract tracing from GnRH neurons in the transgenic mouse, indicate that the dominant populations of ERalpha-expressing neuronal afferents to GnRH neurons reside in the anteroventral periventricular, median preoptic and periventricular preoptic nuclei. Together these estrogen-sensitive afferents to GnRH neurons form a periventricular continuum that can be referred to as rostral periventricular area of the third ventricle (RP3V) neurons. The neurochemical identity of some RP3V neurons has been determined and there is mounting evidence for important roles of glutamate, GABA, kisspeptin and neurotensin-expressing RP3V neurons in estrogen positive feedback. The definition of the key cluster of estrogen-sensitive neurons responsible for activating the GnRH neurons to evoke the GnRH surge (and ovulation) should be of substantial value to on-going efforts to understand the molecular and cellular basis of the estrogen positive feedback mechanism.
Figures
References
-
- Akema T, Praputpittaya C, Kimura F. Effects of preoptic microinjection of neurotensin on luteinizing hormone secretion in unanesthetized ovariectomized rats with or without estrogen priming. Neuroendocrinology. 1987;46:345–349. - PubMed
-
- Alexander MJ, Leeman SE. Estrogen-inducible neurotensin immunoreactivity in the preoptic area of the female rat. Journal of Comparative Neurology. 1994;345:496–509. - PubMed
-
- Alexander MJ, Mahoney PD, Ferris CG, Carraway RE, Leeman SE. Evidence that neurotensin participates in the central regulation of the preovulatory surge of luteinizing hormone in the rat. Endocrinology. 1989;124:783–788. - PubMed
-
- Blache D, Fabre-Nys CJ, Venier G. Ventromedial hypothalamus as a target for oestradiol action on proceptivity, receptivity and luteinizing hormone surge of the ewe. Brain Research. 1991;546:241–249. - PubMed
-
- Bloch GJ, Kurth SM, Akesson TR, Micevych PE. Estrogen-concentrating cells within cell groups of the medial preoptic area: sex differences and co-localization with galanin- immunoreactive cells. Brain Research. 1992;595:301–308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

