Oxidized phospholipids as potential novel drug targets
- PMID: 17604316
- PMCID: PMC2025674
- DOI: 10.1529/biophysj.107.103887
Oxidized phospholipids as potential novel drug targets
Abstract
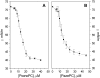
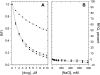
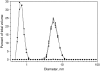
The interactions of three therapeutic agents, viz. the antipsychotics HPD and CPZ, and the antineoplastic anthracycline DOX, with oxidatively modified phospholipids were studied by monitoring the quenching of fluorescence of an incorporated pyrene-labeled lipid derivative. All three drugs bound avidly to the two oxidized PCs bearing either an aldehyde or carboxylic function at the end of the sn-2 nonanoyl chain, with the highest affinity measured between CPZ and the latter oxidized lipid. Subsequent dissociation of the above drugs from the oxidized lipids by DNA, acidic phospholipids, and NaCl revealed the binding of these drugs with the aldehyde lipid to be driven by hydrophobicity similarly to their binding to lysophosphatidylcholine, whereas a significant contribution of electrostatics was evident for the lipid with the carboxylic moiety. These results connect to previous experimental data, demonstrating the induction by these drugs of oxidative stress and binding to membrane phospholipids. These issues are elaborated with reference to their clinical use and side effects.
Figures




Similar articles
-
Comparison of the effects of clozapine, chlorpromazine, and haloperidol on membrane lateral heterogeneity.Chem Phys Lipids. 2001 Aug;112(2):151-63. doi: 10.1016/s0009-3084(01)00175-x. Chem Phys Lipids. 2001. PMID: 11551538
-
Oxidized phospholipid based pH sensitive micelles for delivery of anthracyclines to resistant leukemia cells in vitro.Int J Pharm. 2012 Jan 17;422(1-2):409-17. doi: 10.1016/j.ijpharm.2011.10.029. Epub 2011 Oct 20. Int J Pharm. 2012. PMID: 22037443
-
Spectral analysis of doxorubicin accumulation and the indirect quantification of its DNA intercalation.Eur J Pharm Biopharm. 2010 Nov;76(3):514-24. doi: 10.1016/j.ejpb.2010.07.008. Epub 2010 Jul 16. Eur J Pharm Biopharm. 2010. PMID: 20638475
-
Chlorpromazine binding to Na+, K+-ATPase and photolabeling: involvement of the ouabain site monitored by fluorescence.Photochem Photobiol. 2007 Jul-Aug;83(4):914-9. doi: 10.1111/j.1751-1097.2007.00077.x. Photochem Photobiol. 2007. PMID: 17645663
-
1-Palmitoyl-2-(9'-oxononanoyl)-sn-glycero-3-phosphocholine, an oxidized phospholipid, accelerates Finnish type familial gelsolin amyloidosis in vitro.Biochemistry. 2011 Jun 7;50(22):4877-89. doi: 10.1021/bi200195s. Epub 2011 May 13. Biochemistry. 2011. PMID: 21545139
Cited by
-
Impact of Truncated Oxidized Phosphatidylcholines on Phospholipase A2 Activity in Mono- and Polyunsaturated Biomimetic Vesicles.Int J Mol Sci. 2023 Jul 6;24(13):11166. doi: 10.3390/ijms241311166. Int J Mol Sci. 2023. PMID: 37446342 Free PMC article.
-
Lipid gymnastics: evidence of complete acyl chain reversal in oxidized phospholipids from molecular simulations.Biophys J. 2009 Apr 8;96(7):2734-43. doi: 10.1016/j.bpj.2009.01.007. Biophys J. 2009. PMID: 19348756 Free PMC article.
-
Effect of thioridazine on antioxidant status of HEMn-DP melanocytes.Naunyn Schmiedebergs Arch Pharmacol. 2015 Oct;388(10):1097-104. doi: 10.1007/s00210-015-1144-z. Epub 2015 Jun 24. Naunyn Schmiedebergs Arch Pharmacol. 2015. PMID: 26105001 Free PMC article.
-
EPR spectroscopy of chlorpromazine-induced free radical formation in normal human melanocytes.Eur Biophys J. 2015 Jul;44(5):359-65. doi: 10.1007/s00249-015-1029-6. Epub 2015 May 16. Eur Biophys J. 2015. PMID: 25981866 Free PMC article.
-
Lipid oxidation controls peptide self-assembly near membranes through a surface attraction mechanism.Chem Sci. 2023 Mar 2;14(14):3730-3741. doi: 10.1039/d3sc00159h. eCollection 2023 Apr 5. Chem Sci. 2023. PMID: 37035708 Free PMC article.
References
-
- Mouritsen, O. G. 2005. Life as a Matter of Fat: The Emerging Science of Lipidomics. Springer-Verlag, Berlin, Heidelberg.
-
- Jutila, A., M. Rytömaa, and P. K. J. Kinnunen. 1998. Detachment of cytochrome c by cationic drugs from membranes containing acidic phospholipids: comparison of lidocaine, propranolol, and gentamycin. Mol. Pharmacol. 54:722–732. - PubMed
-
- Mouritsen, O. G., and P. K. J. Kinnunen. 1996. Role of lipid organization and dynamics for membrane functionality. In Biological Membranes: Role of Lipid Organization and Dynamics for Membrane Functionality. K. M. Merz and B. Roux, editors. Birkhäuser, Boston. 463–502.
-
- Kinnunen, P. K. J. 1991. On the principles of functional ordering in biological membranes. Chem. Phys. Lipids. 57:375–399. - PubMed
-
- Söderlund, T., J. Y. A. Lehtonen, and P. K. J. Kinnunen. 1999. Interaction of cyclosporin A with phospholipid membranes. Mol. Pharmacol. 55:32–38. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

