Building the perfect parasite: cell division in apicomplexa
- PMID: 17604449
- PMCID: PMC1904476
- DOI: 10.1371/journal.ppat.0030078
Building the perfect parasite: cell division in apicomplexa
Abstract
Apicomplexans are pathogens responsible for malaria, toxoplasmosis, and crytposporidiosis in humans, and a wide range of livestock diseases. These unicellular eukaryotes are stealthy invaders, sheltering from the immune response in the cells of their hosts, while at the same time tapping into these cells as source of nutrients. The complexity and beauty of the structures formed during their intracellular development have made apicomplexans the darling of electron microscopists. Dramatic technological progress over the last decade has transformed apicomplexans into respectable genetic model organisms. Extensive genomic resources are now available for many apicomplexan species. At the same time, parasite transfection has enabled researchers to test the function of specific genes through reverse and forward genetic approaches with increasing sophistication. Transfection also introduced the use of fluorescent reporters, opening the field to dynamic real time microscopic observation. Parasite cell biologists have used these tools to take a fresh look at a classic problem: how do apicomplexans build the perfect invasion machine, the zoite, and how is this process fine-tuned to fit the specific niche of each pathogen in this ancient and very diverse group? This work has unearthed a treasure trove of novel structures and mechanisms that are the focus of this review.
Conflict of interest statement
Figures

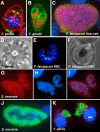

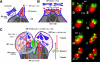
References
-
- Sibley LD. Intracellular parasite invasion strategies. Science. 2004;304:248–253. - PubMed
-
- Dobrowolski JM, Sibley LD. Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell. 1996;84:933–939. - PubMed
-
- Meissner M, Schluter D, Soldati D. Role of Toxoplasma gondii myosin A in powering parasite gliding and host cell invasion. Science. 2002;298:837–840. - PubMed
-
- Carruthers V, Boothroyd JC. Pulling together: An integrated model of Toxoplasma cell invasion. Curr Opin Microbiol. 2006;10:83–89. - PubMed
-
- Cowman AF, Crabb BS. Invasion of red blood cells by malaria parasites. Cell. 2006;124:755–766. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

