Characterization of reemerging chikungunya virus
- PMID: 17604450
- PMCID: PMC1904475
- DOI: 10.1371/journal.ppat.0030089
Characterization of reemerging chikungunya virus
Abstract
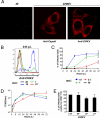
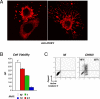
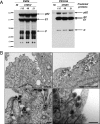
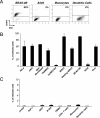
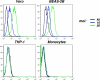
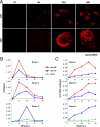
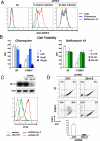
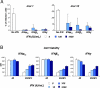
An unprecedented epidemic of chikungunya virus (CHIKV) infection recently started in countries of the Indian Ocean area, causing an acute and painful syndrome with strong fever, asthenia, skin rash, polyarthritis, and lethal cases of encephalitis. The basis for chikungunya disease and the tropism of CHIKV remain unknown. Here, we describe the replication characteristics of recent clinical CHIKV strains. Human epithelial and endothelial cells, primary fibroblasts and, to a lesser extent, monocyte-derived macrophages, were susceptible to infection and allowed viral production. In contrast, CHIKV did not replicate in lymphoid and monocytoid cell lines, primary lymphocytes and monocytes, or monocyte-derived dendritic cells. CHIKV replication was cytopathic and associated with an induction of apoptosis in infected cells. Chloroquine, bafilomycin-A1, and short hairpin RNAs against dynamin-2 inhibited viral production, indicating that viral entry occurs through pH-dependent endocytosis. CHIKV was highly sensitive to the antiviral activity of type I and II interferons. These results provide a general insight into the interaction between CHIKV and its mammalian host.
Conflict of interest statement
Figures
References
-
- Strauss E, Strauss J. Structure and replication of the alphavirus genome. New York: Plenum Press; 1986. pp. 35–90.
-
- Peters C, Dalrymple J. Alphaviruses. In: Fields BN, Knipe DM, editors. Fields virology. 2nd edition. New York: Raven Press; 1990. pp. 713–761.
-
- Bodenmann P, Genton B. Chikungunya: An epidemic in real time. Lancet. 2006;368:258. - PubMed
-
- Pialoux G, Gauzere BA, Jaureguiberry S, Strobel M. Chikungunya, an epidemic arbovirosis. Lancet Infect Dis. 2007;7:319–327. - PubMed
-
- Schuffenecker I, Iteman I, Michault A, Murri S, Frangeul L, et al. Genome microevolution of chikungunya viruses causing the Indian Ocean outbreak. PLoS Med. 2006;3:e263. doi: 10.1371/journal.pmed.0030263. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

