Sequential polymicrobial infections lead to CNS inflammatory disease: possible involvement of bystander activation in heterologous immunity
- PMID: 17604850
- PMCID: PMC1987327
- DOI: 10.1016/j.jneuroim.2007.05.012
Sequential polymicrobial infections lead to CNS inflammatory disease: possible involvement of bystander activation in heterologous immunity
Abstract
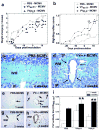
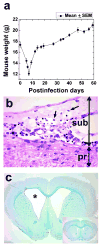
VV(PLP) is a recombinant vaccinia virus (VV) encoding myelin proteolipid protein (PLP) that has been used to investigate molecular mimicry and autoimmunity. Since virus infections can cause bystander activation, mice were first infected with VV(PLP), and later challenged with wild-type VV, lymphocytic choriomeningitis virus (LCMV), or murine cytomegalovirus (MCMV). Among the VV(PLP)-primed mice, only MCMV challenge induced significant Ki-67(+), CD3(+)T cell infiltration into the central nervous system (CNS) with a mild PLP antibody response. While MCMV alone caused no CNS disease, control VV-infected mice followed with MCMV developed mild CNS inflammation. Thus, heterologous virus infections can induce CNS pathology.
Figures




Similar articles
-
Dynamics of memory T cell proliferation under conditions of heterologous immunity and bystander stimulation.J Immunol. 2002 Jul 1;169(1):90-8. doi: 10.4049/jimmunol.169.1.90. J Immunol. 2002. PMID: 12077233
-
Heterologous Immunity and Persistent Murine Cytomegalovirus Infection.J Virol. 2017 Jan 3;91(2):e01386-16. doi: 10.1128/JVI.01386-16. Print 2017 Jan 15. J Virol. 2017. PMID: 27807227 Free PMC article.
-
Protective heterologous antiviral immunity and enhanced immunopathogenesis mediated by memory T cell populations.J Exp Med. 1998 Nov 2;188(9):1705-15. doi: 10.1084/jem.188.9.1705. J Exp Med. 1998. PMID: 9802982 Free PMC article.
-
Multiple sclerosis and virus induced immune responses: autoimmunity can be primed by molecular mimicry and augmented by bystander activation.Autoimmunity. 2006 Feb;39(1):9-19. doi: 10.1080/08916930500484799. Autoimmunity. 2006. PMID: 16455578 Review.
-
T cell response in experimental autoimmune encephalomyelitis (EAE): role of self and cross-reactive antigens in shaping, tuning, and regulating the autopathogenic T cell repertoire.Annu Rev Immunol. 2002;20:101-23. doi: 10.1146/annurev.immunol.20.081701.141316. Epub 2001 Oct 4. Annu Rev Immunol. 2002. PMID: 11861599 Review.
Cited by
-
Experimental autoimmune encephalomyelitis as a testing paradigm for adjuvants and vaccines.Vaccine. 2011 Apr 12;29(17):3356-62. doi: 10.1016/j.vaccine.2010.08.103. Epub 2010 Sep 16. Vaccine. 2011. PMID: 20850537 Free PMC article. Review.
-
Molecular mimicry as a mechanism of autoimmune disease.Clin Rev Allergy Immunol. 2012 Feb;42(1):102-11. doi: 10.1007/s12016-011-8294-7. Clin Rev Allergy Immunol. 2012. PMID: 22095454 Free PMC article. Review.
-
Possible role of interleukin-17 in a prime/challenge model of multiple sclerosis.J Neurovirol. 2012 Dec;18(6):471-8. doi: 10.1007/s13365-012-0125-y. Epub 2012 Sep 19. J Neurovirol. 2012. PMID: 22991336 Free PMC article.
-
CMV infection attenuates the disease course in a murine model of multiple sclerosis.PLoS One. 2012;7(2):e32767. doi: 10.1371/journal.pone.0032767. Epub 2012 Feb 29. PLoS One. 2012. PMID: 22393447 Free PMC article.
-
Studies in the modulation of experimental autoimmune encephalomyelitis.J Neuroimmune Pharmacol. 2010 Jun;5(2):168-75. doi: 10.1007/s11481-010-9215-x. Epub 2010 Apr 17. J Neuroimmune Pharmacol. 2010. PMID: 20401539 Free PMC article. Review.
References
-
- Alotaibi S, Kennedy J, Tellier R, Stephens D, Banwell B. Epstein-Barr virus in pediatric multiple sclerosis. JAMA. 2004;291:1875–1879. - PubMed
-
- Banwell BL. Pediatric multiple sclerosis. Curr Neurol Neurosci Rep. 2004;4:245–252. - PubMed
-
- Barnett LA, Whitton JL, Wada Y, Fujinami RS. Enhancement of autoimmune disease using recombinant vaccinia virus encoding myelin proteolipid protein. J Neuroimmunol. 1993;44:15–25. published erratum appears in J. Neuroimmunol. 48:120, 1993. - PubMed
-
- Biron CA. Initial and innate responses to viral infections--pattern setting in immunity or disease. Curr Opin Microbiol. 1999;2:374–381. - PubMed
-
- Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: Function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

