Function and regulation in MAPK signaling pathways: lessons learned from the yeast Saccharomyces cerevisiae
- PMID: 17604854
- PMCID: PMC2031910
- DOI: 10.1016/j.bbamcr.2007.05.003
Function and regulation in MAPK signaling pathways: lessons learned from the yeast Saccharomyces cerevisiae
Abstract
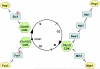
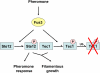
Signaling pathways that activate different mitogen-activated protein kinases (MAPKs) elicit many of the responses that are evoked in cells by changes in certain environmental conditions and upon exposure to a variety of hormonal and other stimuli. These pathways were first elucidated in the unicellular eukaryote Saccharomyces cerevisiae (budding yeast). Studies of MAPK pathways in this organism continue to be especially informative in revealing the molecular mechanisms by which MAPK cascades operate, propagate signals, modulate cellular processes, and are controlled by regulatory factors both internal to and external to the pathways. Here we highlight recent advances and new insights about MAPK-based signaling that have been made through studies in yeast, which provide lessons directly applicable to, and that enhance our understanding of, MAPK-mediated signaling in mammalian cells.
Figures
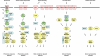

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

