Dexamethasone to prevent postextubation airway obstruction in adults: a prospective, randomized, double-blind, placebo-controlled study
- PMID: 17605780
- PMCID: PMC2206529
- DOI: 10.1186/cc5957
Dexamethasone to prevent postextubation airway obstruction in adults: a prospective, randomized, double-blind, placebo-controlled study
Abstract
Introduction: Prophylactic steroid therapy to reduce the occurrence of postextubation laryngeal edema is controversial. Only a limited number of prospective trials involve adults in an intensive care unit. The purpose of this study was to ascertain whether administration of multiple doses of dexamethasone to critically ill, intubated patients reduces or prevents the occurrence of postextubation airway obstruction. Another specific objective of our study was to investigate whether an after-effect (that is, a transient lingering benefit) exists 24 hours after the discontinuation of dexamethasone.
Methods: A randomized, placebo-controlled, double-blind trial was conducted in an adult medical intensive care unit of a tertiary care hospital. Eighty-six patients who had been intubated for more than 48 hours with a cuff leak volume (CLV) of less than 110 ml and who met weaning criteria were randomly assigned to receive either dexamethasone (5 mg; n = 43) or placebo (normal saline; n = 43) every six hours for a total of four doses on the day preceding extubation. CLV was measured before the first injection, one hour after each injection, and 24 hours after the fourth injection. Extubation was carried out 24 hours after the last injection. Postextubation obstruction (defined as the presence of stridor) was recorded within 48 hours of extubation.
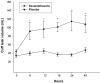
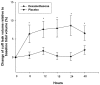
Results: Administration of dexamethasone during the 24-hour period preceding extubation resulted in a statistically significant increase in the CLV (p < 0.05). The significant increase of CLV and change of CLV relative to baseline tidal volume (percentage) occurred not only throughout the treatment period, but also 24 hours after the last dexamethasone injection. The incidence of postextubation stridor was significantly lower in the dexamethasone group than in the placebo group (10% [4/40] versus 27.5% [11/40]; p = 0.037), whereas there was no significant difference in reintubation rate between the two groups (2.5% [1/40] versus 5% [2/40]; p = 0.561).
Conclusion: Prophylactic administration of multiple-dose dexamethasone is effective in reducing the incidence of postextubation stridor in adult patients at high risk for postextubation laryngeal edema. The after-effect of dexamethasone may validate the reduced incidence of postextubation stridor after multiple doses of dexamethasone.
Trial registration: NCT00452062.
Figures

Comment in
-
Corticosteroids to prevent postextubation upper airway obstruction: the evidence mounts.Crit Care. 2007;11(4):156. doi: 10.1186/cc5976. Crit Care. 2007. PMID: 17705879 Free PMC article.
References
-
- Darmon JY, Rauss A, Dreyfuss D, Bleichner G, Elkharrat D, Schlemmer B, Tenaillon A, Brun-Buisson C, Huet Y. Evaluation of risk factors for laryngeal edema after tracheal extubation in adults and its prevention by dexamethasone. A placebo-controlled, double-blind, multicenter study. Anesthesiology. 1992;77:245–251. doi: 10.1097/00000542-199208000-00004. - DOI - PubMed
-
- Demling RH, Read T, Lind LJ, Flanagan HL. Incidence and morbidity of extubation failure in surgical intensive care patients. Crit Care Med. 1988;16:573–577. - PubMed
-
- Esteban A, Alia I, Tobin MJ, Gil A, Gordo F, Vallverdu I, Blanch L, Bonet A, Vazquez A, de Pablo R, et al. Effect of spontaneous breathing trial duration on outcome of attempts to discontinue mechanical ventilation. Spanish Lung Failure Collaborative Group. Am J Respir Crit Care Med. 1999;159:512–518. - PubMed
-
- Epstein SK, Ciubotaru RL, Wong JB. Effect of failed extubation on the outcome of mechanical ventilation. Chest. 1997;112:186–192. - PubMed
-
- Torres A, Gatell JM, Aznar E, el-Ebiary M, Puig de la Bellacasa J, Gonzalez J, Ferrer M, Rodriguez-Roisin R. Reintubation increases the risk of nosocomial pneumonia in patients needing mechanical ventilation. Am J Respir Crit Care Med. 1995;152:137–141. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

