Dominant inhibition of Fas ligand-mediated apoptosis due to a heterozygous mutation associated with autoimmune lymphoproliferative syndrome (ALPS) Type Ib
- PMID: 17605793
- PMCID: PMC1931585
- DOI: 10.1186/1471-2350-8-41
Dominant inhibition of Fas ligand-mediated apoptosis due to a heterozygous mutation associated with autoimmune lymphoproliferative syndrome (ALPS) Type Ib
Abstract
Background: Autoimmune lymphoproliferative syndrome (ALPS) is a disorder of lymphocyte homeostasis and immunological tolerance due primarily to genetic defects in Fas (CD95/APO-1; TNFRSF6), a cell surface receptor that regulates apoptosis and its signaling apparatus.
Methods: Fas ligand gene mutations from ALPS patients were identified through cDNA and genomic DNA sequencing. Molecular and biochemical assessment of these mutant Fas ligand proteins were carried out by expressing the mutant FasL cDNA in mammalian cells and analysis its effects on Fas-mediated programmed cell death.
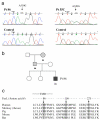
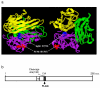
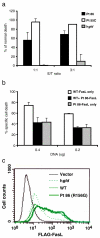
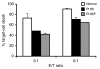
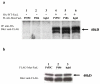
Results: We found an ALPS patient that harbored a heterozygous A530G mutation in the FasL gene that replaced Arg with Gly at position 156 in the protein's extracellular Fas-binding region. This produced a dominant-interfering FasL protein that bound to the wild-type FasL protein and prevented it from effectively inducing apoptosis.
Conclusion: Our data explain how a naturally occurring heterozygous human FasL mutation can dominantly interfere with normal FasL apoptotic function and lead to an ALPS phenotype, designated Type Ib.
Figures

References
-
- Straus SE, Sneller M, Lenardo MJ, Puck JM, Strober W. An inherited disorder of lymphocyte apoptosis: the autoimmune lymphoproliferative syndrome. Ann Intern Med. 1999;130:591–601. - PubMed
-
- Oliveira JB, Bidère N, Niemela J, Zheng LX, Sakai K, Puro C, Danner R, Barb J, Munson P, Puck J, Dale J, Straus S, Fleisher TA, Lenardo MJ. NRAS mutation causes a human autoimmune lymphoproliferative syndrome. Proc Natl Acad Sci USA. 2007;104:8953–8958. doi: 10.1073/pnas.0702975104. - DOI - PMC - PubMed
-
- Puck JM, Straus SE, Le Deist F, Rieux-Laucat F, Fischer A. Inherited Disorders with Autoimmune and Defective Lymphocyte Regulation. In: Ochs H, Smith CIE, Puck JM, editor. Primary Immunodeficiency Diseases: A Molecular and Genetic Approach. New York: Oxford University Press;; 1999. pp. 339–352.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

