Spatio-temporal expression of MMP-2, MMP-9 and tissue kallikrein in uteroplacental units of the pregnant guinea-pig (Cavia porcellus)
- PMID: 17605824
- PMCID: PMC1934362
- DOI: 10.1186/1477-7827-5-27
Spatio-temporal expression of MMP-2, MMP-9 and tissue kallikrein in uteroplacental units of the pregnant guinea-pig (Cavia porcellus)
Abstract
Background: In humans trophoblast invasion and vascular remodeling are critical to determine the fate of pregnancy. Since guinea-pigs share with women an extensive migration of the trophoblasts through the decidua and uterine arteries, and a haemomonochorial placenta, this species was used to evaluate the spatio-temporal expression of three enzymes that have been associated to trophoblast invasion, MMP-2, MMP-9 and tissue kallikrein (K1).
Methods: Uteroplacental units were collected from early to term pregnancy. MMP-2, MMP-9 and K1 were analysed by immunohistochemistry and Western blot. The activities of MMP-2 and MMP-9 were assessed by gelatin zymography.
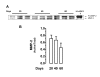
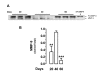
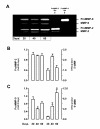
Results: Immunoreactive MMP-2, MMP-9 and K1 were detected in the subplacenta, interlobar and labyrinthine placenta, syncytial sprouts and syncytial streamers throughout pregnancy. In late pregnancy, perivascular or intramural trophoblasts expressed the three enzymes. The intensity of the signal in syncytial streamers was increased in mid and late pregnancy for MMP-2, decreased in late pregnancy for MMP-9, and remained stable for K1. Western blots of placental homogenates at days 20, 40 and 60 of pregnancy identified bands with the molecular weights of MMP-2, MMP-9 and K1. MMP-2 expression remained constant throughout gestation. In contrast, MMP-9 and K1 attained their highest expression during midgestation. Placental homogenates of 20, 40 and 60 days yielded bands of gelatinase activity that were compatible with MMP-2 and MMP-9 activities. ProMMP-2 and MMP-9 activities did not vary along pregnancy, while MMP-2 and MMP-9 increased at 40 and 40-60 days respectively.
Conclusion: The spatio-temporal expression of MMPs and K1 supports a relevant role of these proteins in trophoblast invasion, vascular remodeling and placental angiogenesis, and suggests a functional association between K1 and MMP-9 activation.
Figures






Similar articles
-
Utero-placental expression of angiotensin-(1-7) and ACE2 in the pregnant guinea-pig.Reprod Biol Endocrinol. 2013 Jan 22;11:5. doi: 10.1186/1477-7827-11-5. Reprod Biol Endocrinol. 2013. PMID: 23339712 Free PMC article.
-
Expression and localization of matrix metalloproteinases (MT1-MMP, MMP-2) and tissue inhibitor of metalloproteinase-2 (TIMP-2) during synepitheliochorial placentation of goats (Capra hircus).Placenta. 2004 Nov;25(10):810-9. doi: 10.1016/j.placenta.2004.03.007. Placenta. 2004. PMID: 15451196
-
Distribution patterns of matrix metalloproteinase (MMP)-2 and -9 and their inhibitors (TIMP-1 and TIMP-2) in the human decidua during early pregnancy.Acta Histochem. 2004;106(5):353-62. doi: 10.1016/j.acthis.2004.07.005. Acta Histochem. 2004. PMID: 15530550
-
Comparative matrix metalloproteinase-2 and -9 expression and activity during endotheliochorial and hemochorial trophoblastic invasiveness.Tissue Cell. 2022 Feb;74:101698. doi: 10.1016/j.tice.2021.101698. Epub 2021 Nov 26. Tissue Cell. 2022. PMID: 34871824 Review.
-
Control of MMP-9 expression at the maternal-fetal interface.J Reprod Immunol. 2002 May-Jun;55(1-2):3-10. doi: 10.1016/s0165-0378(01)00142-5. J Reprod Immunol. 2002. PMID: 12062817 Review.
Cited by
-
Vasodilator factors in the systemic and local adaptations to pregnancy.Reprod Biol Endocrinol. 2009 Jul 31;7:79. doi: 10.1186/1477-7827-7-79. Reprod Biol Endocrinol. 2009. PMID: 19646248 Free PMC article. Review.
-
Angiogenic, hyperpermeability and vasodilator network in utero-placental units along pregnancy in the guinea-pig (Cavia porcellus).Reprod Biol Endocrinol. 2008 Mar 27;6:13. doi: 10.1186/1477-7827-6-13. Reprod Biol Endocrinol. 2008. PMID: 18371207 Free PMC article.
-
Administration of angiotensin II and a bradykinin B2 receptor blocker in midpregnancy impairs gestational outcome in guinea pigs.Reprod Biol Endocrinol. 2014 Jun 4;12:49. doi: 10.1186/1477-7827-12-49. Reprod Biol Endocrinol. 2014. PMID: 24893657 Free PMC article.
-
Dietary inulin supplementation in early gestation regulates uterine fluid exosomes and angiogenesis to improve embryo implantation in sows.J Anim Sci Biotechnol. 2025 Aug 5;16(1):111. doi: 10.1186/s40104-025-01247-0. J Anim Sci Biotechnol. 2025. PMID: 40764604 Free PMC article.
-
Utero-placental expression of angiotensin-(1-7) and ACE2 in the pregnant guinea-pig.Reprod Biol Endocrinol. 2013 Jan 22;11:5. doi: 10.1186/1477-7827-11-5. Reprod Biol Endocrinol. 2013. PMID: 23339712 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

