Systematic review identifies number of strategies important for retaining study participants
- PMID: 17606170
- PMCID: PMC1997303
- DOI: 10.1016/j.jclinepi.2006.11.023
Systematic review identifies number of strategies important for retaining study participants
Abstract
Objective: Loss to follow-up threatens internal and external validity yet little research has examined ways to limit participant attrition. We conducted a systematic review of studies with a primary focus on strategies to retain participants in health care research.
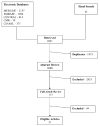
Study design and settings: We completed searches of PubMed, CINAHL, CENTRAL, Cochrane Methodology Register, and EMBASE (August 2005). We also examined reference lists of eligible articles and relevant reviews. A data-driven thematic analysis of the retention strategies identified common themes.
Results: We retrieved 3,068 citations, 21 studies were eligible for inclusion. We abstracted 368 strategies and from these identified 12 themes. The studies reported a median of 17 strategies across a median of six themes. The most commonly reported strategies were systematic methods of participant contact and scheduling. Studies with retention rates lower than the mean rate (86%) reported fewer strategies. There was no difference in the number of different themes used.
Conclusion: Available evidence suggests that investigators should consider using a number of retention strategies across several themes to maximize the retention of participants. Further research, including explicit evaluation of the effectiveness of different strategies, is needed.
Figures
References
-
- Szklo M, Nieto FJ. Epidemiology - Beyond the basics. Boston, MA: Jones and Bartlett Publishers; 2004.
-
- Friedman LM, Furberg CD, DeMets DL. Fundamentals of Clinical Trials. St Louis: Mosby; 1995.
-
- Cassidy EL, Baird E, Sheikh JI. Recruitment and retention of elderly patients in clinical trials: issues and strategies. Am J Geriatr Psychiatry. 2001 Spring;9(2):136–40. - PubMed
-
- Shumaker SA, Dugan E, Bowen DJ. Enhancing adherence in randomized controlled clinical trials. Control Clin Trials. 2000 Oct;21(5 Suppl):226S–32S. - PubMed
-
- Coday M, Boutin-Foster C, Goldman Sher T, Tennant J, Greaney ML, Saunders SD, Somes GW. Strategies for Retaining Study Participants in Behavioral Intervention Trials: Retention Experiences of the NIH Behavior Change Consortium. Ann Behav Med. 2005;29(2 Suppl):55–65. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources