Gastrointestinal dysfunction in mice with a targeted mutation in the gene encoding vasoactive intestinal polypeptide: a model for the study of intestinal ileus and Hirschsprung's disease
- PMID: 17606312
- PMCID: PMC2042583
- DOI: 10.1016/j.peptides.2007.05.006
Gastrointestinal dysfunction in mice with a targeted mutation in the gene encoding vasoactive intestinal polypeptide: a model for the study of intestinal ileus and Hirschsprung's disease
Abstract
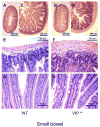
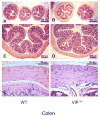
In 1970, Drs. Said and Mutt isolated a novel peptide from porcine intestinal extracts with powerful vasoactive properties, and named it vasoactive intestinal peptide (VIP). Since then, the biological actions of VIP in the gut as well as its signal transduction pathways have been extensively studied. A variety of in vitro and in vivo studies have indicated that VIP, expressed in intrinsic non-adrenergic non-cholinergic (NANC) neurons, is a potent regulator of gastrointestinal (GI) motility, water absorption and ion flux, mucus secretion and immune homeostasis. These VIP actions are believed to be mediated mainly by interactions with highly expressed VPAC(1) receptors and the production of nitric oxide (NO). Furthermore, VIP has been implicated in numerous physiopathological conditions affecting the human gut, including pancreatic endocrine tumors secreting VIP (VIPomas), insulin-dependent diabetes, Hirschsprung's disease, and inflammatory bowel syndromes such as Crohn's disease and ulcerative colitis. To further understand the physiological roles of VIP on the GI tract, we have begun to analyze the anatomical and physiological phenotype of C57BL/6 mice lacking the VIP gene. Herein, we demonstrate that the overall intestinal morphology and light microscopic structure is significantly altered in VIP(-/-) mice. Macroscopically there is an overall increase in weight, and decrease in length of the bowel compared to wild type (WT) controls. Microscopically, the phenotype was characterized by thickening of smooth muscle layers, increased villi length, and higher abundance of goblet cells. Alcian blue staining indicated that the latter cells were deficient in mucus secretion in VIP(-/-) mice. The differences became more pronounced from the duodenum to the distal jejunum or ileum of the small bowel but, became much less apparent or absent in the colon with the exception of mucus secretion defects. Further examination of the small intestine revealed larger axonal trunks and unusual unstained patches in myenteric plexus. Physiologically, the VIP(-/-) mice showed an impairment in intestinal transit. Moreover, unlike WT C57BL/6 mice, a significant percentage of VIP(-/-) mice died in the first postnatal year with overt stenosis of the gut.
Figures




Similar articles
-
VPAC1 receptors regulate intestinal secretion and muscle contractility by activating cholinergic neurons in guinea pig jejunum.Am J Physiol Gastrointest Liver Physiol. 2014 May 1;306(9):G748-58. doi: 10.1152/ajpgi.00416.2013. Epub 2014 Feb 27. Am J Physiol Gastrointest Liver Physiol. 2014. PMID: 24578344
-
Expression and localization of VPAC1, the major receptor of vasoactive intestinal peptide along the length of the intestine.Am J Physiol Gastrointest Liver Physiol. 2017 Jul 1;313(1):G16-G25. doi: 10.1152/ajpgi.00081.2017. Epub 2017 Apr 6. Am J Physiol Gastrointest Liver Physiol. 2017. PMID: 28385693 Free PMC article.
-
Intestinotrophic glucagon-like peptide-2 (GLP-2) activates intestinal gene expression and growth factor-dependent pathways independent of the vasoactive intestinal peptide gene in mice.Endocrinology. 2012 Jun;153(6):2623-32. doi: 10.1210/en.2012-1069. Epub 2012 Apr 24. Endocrinology. 2012. PMID: 22535770 Free PMC article.
-
Interaction of NO and VIP in gastrointestinal smooth muscle relaxation.Curr Pharm Des. 2004;10(20):2483-97. doi: 10.2174/1381612043383890. Curr Pharm Des. 2004. PMID: 15320758 Review.
-
Recent advances in vasoactive intestinal peptide physiology and pathophysiology: focus on the gastrointestinal system.F1000Res. 2019 Sep 12;8:F1000 Faculty Rev-1629. doi: 10.12688/f1000research.18039.1. eCollection 2019. F1000Res. 2019. PMID: 31559013 Free PMC article. Review.
Cited by
-
Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR review 1.Br J Pharmacol. 2012 May;166(1):4-17. doi: 10.1111/j.1476-5381.2012.01871.x. Br J Pharmacol. 2012. PMID: 22289055 Free PMC article. Review.
-
The neuropeptide VIP confers anticipatory mucosal immunity by regulating ILC3 activity.Nat Immunol. 2020 Feb;21(2):168-177. doi: 10.1038/s41590-019-0567-y. Epub 2019 Dec 23. Nat Immunol. 2020. PMID: 31873294
-
Dlx1/2 mice have abnormal enteric nervous system function.JCI Insight. 2020 Feb 27;5(4):e131494. doi: 10.1172/jci.insight.131494. JCI Insight. 2020. PMID: 32017713 Free PMC article.
-
Mice deficient in both pituitary adenylyl cyclase-activating polypeptide and vasoactive intestinal peptide survive, but display growth retardation and sex-dependent early death.J Mol Neurosci. 2008 Nov;36(1-3):200-7. doi: 10.1007/s12031-008-9085-3. Epub 2008 May 20. J Mol Neurosci. 2008. PMID: 18491042
-
Functional role of vasoactive intestinal polypeptide in inhibitory motor innervation in the mouse internal anal sphincter.J Physiol. 2013 Mar 15;591(6):1489-506. doi: 10.1113/jphysiol.2012.247684. Epub 2013 Jan 21. J Physiol. 2013. PMID: 23339175 Free PMC article.
References
-
- Abad C, Martinez C, Juarranz MG, Arranz A, Leceta J, Delgado M, Gomariz RP. Therapeutic effects of vasoactive intestinal peptide in the trinitrobenzene sulfonic acid mice model of Crohn’s disease. Gastroenterology. 2003;124:961–71. - PubMed
-
- Barbezat GO, Grossman MI. Intestinal secretion: stimulation by peptides. Science. 1971;174:422–4. - PubMed
-
- Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K, Parker AR, Shimada Y, Eshleman JR, Watkins DN, Beachy PA. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846–51. - PubMed
-
- Binder HJ, Lemp GF, Gardner JD. Receptors for vasoactive intestinal peptide and secretin on small intestinal epithelial cells. Am J Physiol. 1980;238:G190–6. - PubMed
-
- Colwell CS, Michel S, Itri J, Rodriguez W, Tam J, Lelievre V, Hu Z, Liu X, Waschek JA. Disrupted circadian rhythms in VIP- and PHI-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R939–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

