Neonatal (+)-methamphetamine increases brain derived neurotrophic factor, but not nerve growth factor, during treatment and results in long-term spatial learning deficits
- PMID: 17606327
- PMCID: PMC2756096
- DOI: 10.1016/j.psyneuen.2007.05.004
Neonatal (+)-methamphetamine increases brain derived neurotrophic factor, but not nerve growth factor, during treatment and results in long-term spatial learning deficits
Abstract
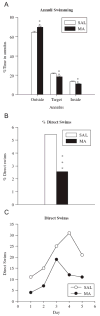
In this study, brain derived neurotrophic factor (BDNF) and nerve growth factor (NGF) were examined at five time points [postnatal day (P)11, 15, 20, 21, and 68 (the latter with or without behavioral testing)] during and after P11-20 (+)-methamphetamine (MA) (10 mg/kg 4 x day) treatment. BDNF in MA-treated animals was elevated on P15 and P20 in the hippocampus but not in the hypothalamus and was unchanged on P11 and P21. On P68 (1 h after Morris water maze testing) MA-treated offspring showed a trend toward higher levels of BDNF in the hippocampus than saline-treated animals. MA treatment increased NGF levels in the hippocampus but only on P20. No effect of MA treatment was observed in the elevated zero maze. MA-treated offspring had increased latencies, cumulative distances, path lengths, and first bearings in the Morris water maze. The findings indicate that early MA exposure induces hippocampal BDNF increases that precede the later emergence of spatial learning deficits.
Conflict of interest statement
Figures


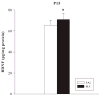

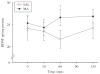
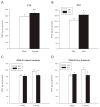
References
-
- Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. - PubMed
-
- Beiko J, Lander R, Hampson E, Boon F, Cain DP. Contribution of sex differences in the acute stress response to sex differences in water maze performance in the rat. Behav Brain Res. 2004;151:239–253. - PubMed
-
- Bekinschtein P, Cammarota M, Igaz LM, Bevilaqua LR, Izquierdo I, Medina JH. Persistence of long-term memory storage requires a late protein synthesis-and. Neuron. 2007;53:261–277. - PubMed
-
- Bimonte-Nelson HA, Singleton RS, Nelson ME, Eckman CB, Barber J, Scott TY, Granholm AC. Testosterone, but not nonaromatizable dihydrotestosterone, improves working memory and alters nerve growth factor levels in aged male rats. Exp Neurol. 2003;181:301–312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

