Heligmosomoides polygyrus promotes regulatory T-cell cytokine production in the murine normal distal intestine
- PMID: 17606601
- PMCID: PMC1951154
- DOI: 10.1128/IAI.00358-07
Heligmosomoides polygyrus promotes regulatory T-cell cytokine production in the murine normal distal intestine
Abstract
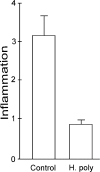
Helminths down-regulate inflammation and may prevent development of several autoimmune illnesses, such as inflammatory bowel disease. We determined if exposure to the duodenal helminth Heligmosomoides polygyrus establishes cytokine pathways in the distal intestine that may protect from intestinal inflammation. Mice received 200 H. polygyrus larvae and were studied 2 weeks later. Lamina propria mononuclear cells (LPMC) were isolated from the terminal ileum for analysis and in vitro experiments. Mice with H. polygyrus were resistant to trinitrobenzenesulfonic acid (TNBS)-induced colitis, a Th1 cytokine-dependent inflammation. Heligmosomoides polygyrus did not change the normal microscopic appearance of the terminal ileum and colon and minimally affected LPMC composition. However, colonization altered LPMC cytokine profiles, blocking gamma interferon (IFN-gamma) and interleukin 12 (IL-12) p40 release but promoting IL-4, IL-5, IL-13, and IL-10 secretion. IL-10 blockade in vitro with anti-IL-10 receptor (IL-10R) monoclonal antibody restored LPMC IFN-gamma and IL-12 p40 secretion. IL-10 blockade in vivo worsened TNBS colitis in H. polygyrus-colonized mice. Lamina propria CD4(+) T cells isolated from colonized mice inhibited IFN-gamma production by splenic T cells from worm-free mice. This inhibition did not require cell contact and was dependent on IL-10. Heligmosomoides polygyrus colonization inhibits Th1 and promotes Th2 and regulatory cytokine production in distant intestinal regions without changing histology or LPMC composition. IL-10 is particularly important for limiting the Th1 response. The T-cell origin of these cytokines demonstrates mucosal regulatory T-cell induction.
Figures
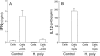


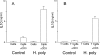

Similar articles
-
Heligmosomoides polygyrus inhibits established colitis in IL-10-deficient mice.Eur J Immunol. 2004 Oct;34(10):2690-8. doi: 10.1002/eji.200324833. Eur J Immunol. 2004. PMID: 15368285
-
Colonization with Heligmosomoides polygyrus suppresses mucosal IL-17 production.J Immunol. 2008 Aug 15;181(4):2414-9. doi: 10.4049/jimmunol.181.4.2414. J Immunol. 2008. PMID: 18684931 Free PMC article.
-
Role of T cell TGF-beta signaling in intestinal cytokine responses and helminthic immune modulation.Eur J Immunol. 2009 Jul;39(7):1870-8. doi: 10.1002/eji.200838956. Eur J Immunol. 2009. PMID: 19544487 Free PMC article.
-
Animal model of Nippostrongylus brasiliensis and Heligmosomoides polygyrus.Curr Protoc Immunol. 2003 Aug;Chapter 19:Unit 19.12. doi: 10.1002/0471142735.im1912s55. Curr Protoc Immunol. 2003. PMID: 18432905 Review.
-
Immune modulation and modulators in Heligmosomoides polygyrus infection.Exp Parasitol. 2012 Sep;132(1):76-89. doi: 10.1016/j.exppara.2011.08.011. Epub 2011 Aug 22. Exp Parasitol. 2012. PMID: 21875581 Free PMC article. Review.
Cited by
-
Helminth Therapy: Advances in the use of Parasitic Worms Against Inflammatory Bowel Diseases and its Challenges.Helminthologia. 2018 Jan 27;55(1):1-11. doi: 10.1515/helm-2017-0048. eCollection 2018 Mar. Helminthologia. 2018. PMID: 31662622 Free PMC article.
-
Btn2a2 Regulates ILC2-T Cell Cross Talk in Type 2 Immune Responses.Front Immunol. 2022 Jan 25;13:757436. doi: 10.3389/fimmu.2022.757436. eCollection 2022. Front Immunol. 2022. PMID: 35145516 Free PMC article.
-
TGF-β mimic proteins form an extended gene family in the murine parasite Heligmosomoides polygyrus.Int J Parasitol. 2018 Apr;48(5):379-385. doi: 10.1016/j.ijpara.2017.12.004. Epub 2018 Mar 3. Int J Parasitol. 2018. PMID: 29510118 Free PMC article.
-
Helminth infections decrease host susceptibility to immune-mediated diseases.J Immunol. 2014 Oct 1;193(7):3239-47. doi: 10.4049/jimmunol.1400927. J Immunol. 2014. PMID: 25240019 Free PMC article.
-
Potential treatment of inflammatory bowel disease: a review of helminths therapy.Gastroenterol Hepatol Bed Bench. 2014 Winter;7(1):9-16. Gastroenterol Hepatol Bed Bench. 2014. PMID: 25436093 Free PMC article. Review.
References
-
- Akbari, O., G. J. Freeman, E. H. Meyer, E. A. Greenfield, T. T. Chang, A. H. Sharpe, G. Berry, R. H. DeKruyff, and D. T. Umetsu. 2002. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat. Med. 8:1024-1032. - PubMed
-
- Bandeira-Melo, C., S. A. Perez, R. C. Melo, I. Ghiran, and P. F. Weller. 2003. EliCell assay for the detection of released cytokines from eosinophils. J. Immunol. Methods 276:227-237. - PubMed
-
- Bashir, M. E., P. Andersen, I. J. Fuss, H. N. Shi, and C. Nagler-Anderson. 2002. An enteric helminth infection protects against an allergic response to dietary antigen. J. Immunol. 169:3284-3292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

