Fer-mediated cortactin phosphorylation is associated with efficient fibroblast migration and is dependent on reactive oxygen species generation during integrin-mediated cell adhesion
- PMID: 17606629
- PMCID: PMC1952165
- DOI: 10.1128/MCB.01744-06
Fer-mediated cortactin phosphorylation is associated with efficient fibroblast migration and is dependent on reactive oxygen species generation during integrin-mediated cell adhesion
Abstract
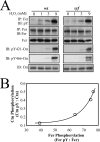
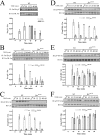
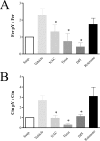
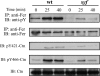
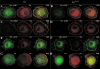
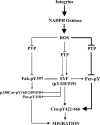
The molecular details linking integrin engagement to downstream cortactin (Ctn) tyrosine phosphorylation are largely unknown. In this report, we show for the first time that Fer and Ctn are potently tyrosine phosphorylated in response to hydrogen peroxide (H2O2) in a variety of cell types. Working with catalytically inactive fer and src/yes/fyn-deficient murine embryonic fibroblasts (ferDR/DR and syf MEF, respectively), we observed that H2O2-induced Ctn tyrosine phosphorylation is primarily dependent on Fer but not Src family kinase (SFK) activity. We also demonstrated for the first time that Fer is activated by fibronectin engagement and, in concert with SFKs, mediates Ctn tyrosine phosphorylation in integrin signaling pathways. Reactive oxygen species (ROS) scavengers or the NADPH oxidase inhibitor, diphenylene iodonium, attenuated integrin-induced Fer and Ctn tyrosine phosphorylation. Taken together, these findings provide novel genetic evidence that a ROS-Fer signaling arm contributes to SFK-mediated Ctn tyrosine phosphorylation in integrin signaling. Lastly, a migration defect in ferDR/DR MEF suggests that integrin signaling through the ROS-Fer-Ctn signaling arm may be linked to mechanisms governing cell motility. These data demonstrate for the first time an oxidative link between integrin adhesion and an actin-binding protein involved in actin polymerization.
Figures




Similar articles
-
Reactive oxygen species as essential mediators of cell adhesion: the oxidative inhibition of a FAK tyrosine phosphatase is required for cell adhesion.J Cell Biol. 2003 Jun 9;161(5):933-44. doi: 10.1083/jcb.200211118. J Cell Biol. 2003. PMID: 12796479 Free PMC article.
-
Specific tyrosine phosphorylation of focal adhesion kinase mediated by Fer tyrosine kinase in suspended hepatocytes.Biochim Biophys Acta. 2009 May;1793(5):781-91. doi: 10.1016/j.bbamcr.2009.01.015. Epub 2009 Feb 5. Biochim Biophys Acta. 2009. PMID: 19339212
-
Src family kinases are required for integrin but not PDGFR signal transduction.EMBO J. 1999 May 4;18(9):2459-71. doi: 10.1093/emboj/18.9.2459. EMBO J. 1999. PMID: 10228160 Free PMC article.
-
Src kinases as therapeutic targets for cancer.Nat Rev Clin Oncol. 2009 Oct;6(10):587-95. doi: 10.1038/nrclinonc.2009.129. Nat Rev Clin Oncol. 2009. PMID: 19787002 Review.
-
Src family protein tyrosine kinases: cooperating with growth factor and adhesion signaling pathways.Curr Opin Cell Biol. 1997 Apr;9(2):187-92. doi: 10.1016/s0955-0674(97)80062-2. Curr Opin Cell Biol. 1997. PMID: 9069259 Review.
Cited by
-
Integrins and cadherins join forces to form adhesive networks.J Cell Sci. 2011 Apr 15;124(Pt 8):1183-93. doi: 10.1242/jcs.064618. J Cell Sci. 2011. PMID: 21444749 Free PMC article. Review.
-
The role of Nox-mediated oxidation in the regulation of cytoskeletal dynamics.Curr Pharm Des. 2015;21(41):6009-22. doi: 10.2174/1381612821666151029112624. Curr Pharm Des. 2015. PMID: 26510432 Free PMC article. Review.
-
Opposing Actions of Fibroblast and Cardiomyocyte Smad3 Signaling in the Infarcted Myocardium.Circulation. 2018 Feb 13;137(7):707-724. doi: 10.1161/CIRCULATIONAHA.117.029622. Epub 2017 Dec 11. Circulation. 2018. PMID: 29229611 Free PMC article.
-
Astrocyte Structural and Molecular Response to Elevated Intraocular Pressure Occurs Rapidly and Precedes Axonal Tubulin Rearrangement within the Optic Nerve Head in a Rat Model.PLoS One. 2016 Nov 28;11(11):e0167364. doi: 10.1371/journal.pone.0167364. eCollection 2016. PLoS One. 2016. PMID: 27893827 Free PMC article.
-
The Fer tyrosine kinase regulates interactions of Rho GDP-Dissociation Inhibitor α with the small GTPase Rac.BMC Biochem. 2010 Dec 1;11:48. doi: 10.1186/1471-2091-11-48. BMC Biochem. 2010. PMID: 21122136 Free PMC article.
References
-
- Agerer, F., S. Lux, A. Michel, M. Rohde, K. Ohlsen, and C. R. Hauck. 2005. Cellular invasion by Staphylococcus aureus reveals a functional link between focal adhesion kinase and cortactin in integrin-mediated internalisation. J. Cell Sci. 118:2189-2200. - PubMed
-
- Allard, P., A. Zoubeidi, L. T. Nguyen, S. Tessier, S. Tanguay, M. Chevrette, A. Aprikian, and S. Chevalier. 2000. Links between Fer tyrosine kinase expression levels and prostate cell proliferation. Mol. Cell. Endocrinol. 159:63-77. - PubMed
-
- Aspenstrom, P. 1997. A Cdc42 target protein with homology to the non-kinase domain of FER has a potential role in regulating the actin cytoskeleton. Curr. Biol. 7:479-487. - PubMed
-
- Bae, Y. S., S. W. Kang, M. S. Seo, I. C. Baines, E. Tekle, P. B. Chock, and S. G. Rhee. 1997. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J. Biol. Chem. 272:217-221. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
