Distinct roles for Syk and ZAP-70 during early thymocyte development
- PMID: 17606633
- PMCID: PMC2118636
- DOI: 10.1084/jem.20070405
Distinct roles for Syk and ZAP-70 during early thymocyte development
Abstract
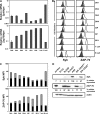
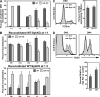
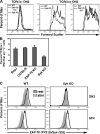
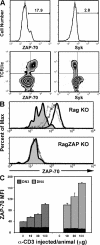
The spleen tyrosine kinase (Syk) and zeta-associated protein of 70 kD (ZAP-70) tyrosine kinases are both expressed during early thymocyte development, but their unique thymic functions have remained obscure. No specific role for Syk during beta-selection has been established, and no role has been described for ZAP-70 before positive selection. We show that Syk and ZAP-70 provide thymocytes with unique and separable fitness advantages during early development. Syk-deficient, but not ZAP-70-deficient, thymocytes are specifically impaired in initial pre-TCR signaling at the double-negative (DN) 3 beta selection stage and show reduced cell-cycle entry. Surprisingly, and despite overlapping expression of both kinases, only ZAP-70 appears to promote sustained pre-TCR/TCR signaling during the DN4, immature single-positive, and double-positive stages of development before thymic selection occurs. ZAP-70 promotes survival and cell-cycle progression of developing thymocytes before positive selection, as also shown by in vivo anti-CD3 treatment of recombinase-activating gene 1-deficient mice. Our results establish a temporal separation of Syk family kinase function during early thymocyte development and a novel role for ZAP-70. We propose that pre-TCR signaling continues during DN4 and later stages, with ZAP-70 dynamically replacing Syk for continued pre-TCR signaling.
Figures
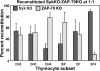


References
-
- Cheng, A.M., B. Rowley, W. Pao, A. Hayday, J.B. Bolen, and T. Pawson. 1995. Syk tyrosine kinase required for mouse viability and B-cell development. Nature. 378:303–306. - PubMed
-
- Turner, M., P.J. Mee, P.S. Costello, O. Williams, A.A. Price, L.P. Duddy, M.T. Furlong, R.L. Geahlen, and V.L. Tybulewicz. 1995. Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature. 378:298–302. - PubMed
-
- Groves, T., P. Smiley, M.P. Cooke, K. Forbush, R.M. Perlmutter, and C.J. Guidos. 1996. Fyn can partially substitute for Lck in T lymphocyte development. Immunity. 5:417–428. - PubMed
-
- van Oers, N.S.C., B. Lowin-Kropf, D. Finlay, K. Connolly, and A. Weiss. 1996. αβ T cell development is abolished in mice lacking both Lck and Fyn protein tyrosine kinases. Immunity. 5:429–436. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

