Cerebellar neurodegeneration in the absence of microRNAs
- PMID: 17606634
- PMCID: PMC2118654
- DOI: 10.1084/jem.20070823
Cerebellar neurodegeneration in the absence of microRNAs
Abstract
Genome-encoded microRNAs (miRNAs) are potent regulators of gene expression. The significance of miRNAs in various biological processes has been suggested by studies showing an important role of these small RNAs in regulation of cell differentiation. However, the role of miRNAs in regulation of differentiated cell physiology is not well established. Mature neurons express a large number of distinct miRNAs, but the role of miRNAs in postmitotic neurons has not been examined. Here, we provide evidence for an essential role of miRNAs in survival of differentiated neurons. We show that conditional Purkinje cell-specific ablation of the key miRNA-generating enzyme Dicer leads to Purkinje cell death. Deficiency in Dicer is associated with progressive loss of miRNAs, followed by cerebellar degeneration and development of ataxia. The progressive neurodegeneration in the absence of Dicer raises the possibility of an involvement of miRNAs in neurodegenerative disorders.
Figures
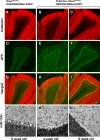



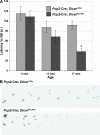
References
-
- Ambros, V. 2004. The functions of animal microRNAs. Nature. 431:350–355. - PubMed
-
- Bartel, D.P. 2004. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 116:281–297. - PubMed
-
- He, L., and G.J. Hannon. 2004. MicroRNAs: small RNAs with a big role in gene regulation. Nat. Rev. Genet. 5:522–531. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

