Cancer vaccines: moving beyond current paradigms
- PMID: 17606707
- PMCID: PMC2536755
- DOI: 10.1158/1078-0432.CCR-07-0588
Cancer vaccines: moving beyond current paradigms
Abstract
The field of cancer vaccines is currently in an active state of preclinical and clinical investigations. Although no therapeutic cancer vaccine has to date been approved by the Food and Drug Administration, several new paradigms are emerging from recent clinical findings both in the use of combination therapy approaches and, perhaps more importantly, in clinical trial design and end point analyses. This article will review recent clinical trials involving several different cancer vaccines from which data are emerging contrasting classic "tumor response" (Response Evaluation Criteria in Solid Tumors) criteria with "patient response" in the manifestation of increased patient survival post-vaccine therapy. Also described are several strategies in which cancer vaccines can be exploited in combination with other agents and therapeutic modalities that are quite unique when compared with "conventional" combination therapies. This is most likely due to the phenomena that (a) cancer vaccines initiate a dynamic immune process that can be exploited in subsequent therapies and (b) both radiation and certain chemotherapeutic agents have been shown to alter the phenotype of tumor cells as to render them more susceptible to T-cell--mediated killing. Consequently, evidence is emerging from several studies in which patient cohorts who first receive a cancer vaccine (as contrasted with control cohorts) benefit clinically from subsequent therapies.
Figures
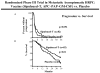




References
-
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. - PubMed
-
- Therasse P, Eisenhauer EA, Verweij J. RECIST revisited: a review of validation studies on tumour assessment. Eur J Cancer. 2006;42:1031–9. - PubMed
-
- Gore ME, Escudier B. Emerging efficacy endpoints for targeted therapies in advanced renal cell carcinoma. Oncology (Williston Park, NY. 2006;20:19–24. - PubMed
-
- Hoos A, Parmiani G, Hege K, et al. A clinical development paradigm for cancer vaccines and related biologics. J Immunother. 2007;30:1–15. - PubMed
-
- Tuma RS. Sometimes size doesn’t matter: reevaluating RECIST and tumor response rate endpoints. J Natl Cancer Inst. 2006;98:1272–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

