The evolution of N-glycan-dependent endoplasmic reticulum quality control factors for glycoprotein folding and degradation
- PMID: 17606910
- PMCID: PMC1905923
- DOI: 10.1073/pnas.0704862104
The evolution of N-glycan-dependent endoplasmic reticulum quality control factors for glycoprotein folding and degradation
Abstract
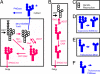
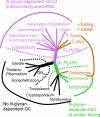
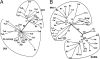
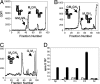
Asn-linked glycans (N-glycans) play important roles in the quality control (QC) of glycoprotein folding in the endoplasmic reticulum (ER) lumen and in ER-associated degradation (ERAD) of proteins by cytosolic proteasomes. A UDP-Glc:glycoprotein glucosyltransferase glucosylates N-glycans of misfolded proteins, which are then bound and refolded by calreticulin and/or calnexin in association with a protein disulfide isomerase. Alternatively, an alpha-1,2-mannosidase (Mns1) and mannosidase-like proteins (ER degradation-enhancing alpha-mannosidase-like proteins 1, 2, and 3) are part of a process that results in the dislocation of misfolded glycoproteins into the cytosol, where proteins are degraded in the proteasome. Recently we found that numerous protists and fungi contain 0-11 sugars in their N-glycan precursors versus 14 sugars in those of animals, plants, fungi, and Dictyostelium. Our goal here was to determine what effect N-glycan precursor diversity has on N-glycan-dependent QC systems of glycoprotein folding and ERAD. N-glycan-dependent QC of folding (UDP-Glc:glycoprotein glucosyltransferase, calreticulin, and/or calnexin) was present and active in some but not all protists containing at least five mannose residues in their N-glycans and was absent in protists lacking Man. In contrast, N-glycan-dependent ERAD appeared to be absent from the majority of protists. However, Trypanosoma and Trichomonas genomes predicted ER degradation-enhancing alpha-mannosidase-like protein and Mns1 orthologs, respectively, each of which had alpha-mannosidase activity in vitro. Phylogenetic analyses suggested that the diversity of N-glycan-dependent QC of glycoprotein folding (and possibly that of ERAD) was best explained by secondary loss. We conclude that N-glycan precursor length has profound effects on N-glycan-dependent QC of glycoprotein folding and ERAD.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
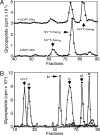


Similar articles
-
Effects of N-glycan precursor length diversity on quality control of protein folding and on protein glycosylation.Semin Cell Dev Biol. 2015 May;41:121-8. doi: 10.1016/j.semcdb.2014.11.008. Epub 2014 Dec 2. Semin Cell Dev Biol. 2015. PMID: 25475176 Free PMC article. Review.
-
N-glycan structure of a short-lived variant of ribophorin I expressed in the MadIA214 glycosylation-defective cell line reveals the role of a mannosidase that is not ER mannosidase I in the process of glycoprotein degradation.Glycobiology. 2001 Jul;11(7):565-76. doi: 10.1093/glycob/11.7.565. Glycobiology. 2001. PMID: 11447136
-
Role of N-oligosaccharide endoplasmic reticulum processing reactions in glycoprotein folding and degradation.Biochem J. 2000 May 15;348 Pt 1(Pt 1):1-13. Biochem J. 2000. PMID: 10794707 Free PMC article. Review.
-
Identification of an Htm1 (EDEM)-dependent, Mns1-independent Endoplasmic Reticulum-associated Degradation (ERAD) pathway in Saccharomyces cerevisiae: application of a novel assay for glycoprotein ERAD.J Biol Chem. 2010 Aug 6;285(32):24324-34. doi: 10.1074/jbc.M109.095919. Epub 2010 May 28. J Biol Chem. 2010. PMID: 20511219 Free PMC article.
-
Quality control of glycoprotein folding and ERAD: the role of N-glycan handling, EDEM1 and OS-9.Histochem Cell Biol. 2017 Feb;147(2):269-284. doi: 10.1007/s00418-016-1513-9. Epub 2016 Nov 1. Histochem Cell Biol. 2017. PMID: 27803995 Review.
Cited by
-
Analysis of glycoprotein processing in the endoplasmic reticulum using synthetic oligosaccharides.Proc Jpn Acad Ser B Phys Biol Sci. 2012;88(2):31-40. doi: 10.2183/pjab.88.31. Proc Jpn Acad Ser B Phys Biol Sci. 2012. PMID: 22314014 Free PMC article. Review.
-
N-Glycosylation at Asn291 Stabilizes TIM-4 and Promotes the Metastasis of NSCLC.Front Oncol. 2022 Mar 31;12:730530. doi: 10.3389/fonc.2022.730530. eCollection 2022. Front Oncol. 2022. PMID: 35433445 Free PMC article.
-
N-glycosylation does not affect the catalytic activity of ricin a chain but stimulates cytotoxicity by promoting its transport out of the endoplasmic reticulum.Traffic. 2012 Nov;13(11):1508-21. doi: 10.1111/j.1600-0854.2012.01404.x. Epub 2012 Sep 7. Traffic. 2012. PMID: 22882900 Free PMC article.
-
Essential roles of the Kar2/BiP molecular chaperone downstream of the UPR pathway in Cryptococcus neoformans.PLoS One. 2013;8(3):e58956. doi: 10.1371/journal.pone.0058956. Epub 2013 Mar 6. PLoS One. 2013. PMID: 23484059 Free PMC article.
-
Evolutionary diversity of social amoebae N-glycomes may support interspecific autonomy.Glycoconj J. 2015 Aug;32(6):345-59. doi: 10.1007/s10719-015-9592-8. Epub 2015 May 19. Glycoconj J. 2015. PMID: 25987342 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

