Hepcidin regulation: ironing out the details
- PMID: 17607352
- PMCID: PMC1904333
- DOI: 10.1172/JCI32701
Hepcidin regulation: ironing out the details
Abstract
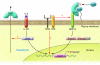
Hepcidin is a peptide hormone secreted by the liver that plays a central role in the regulation of iron homeostasis. Increased hepcidin levels result in anemia while decreased expression is the causative feature in most primary iron overload diseases. Mutations in hemochromatosis type 2 (HFE2), which encodes the protein hemojuvelin (HJV), result in the absence of hepcidin and an early-onset form of iron overload disease. HJV is a bone morphogenetic protein (BMP) coreceptor and HJV mutants have impaired BMP signaling. In this issue of the JCI, Babitt and colleagues show that BMPs are autocrine hormones that induce hepcidin expression (see the related article beginning on page 1933). Administration of a recombinant, soluble form of HJV decreased hepcidin expression and increased serum iron levels by mobilizing iron from splenic stores. These results demonstrate that recombinant HJV may be a useful therapeutic agent for treatment of the anemia of chronic disease, a disorder resulting from high levels of hepcidin expression.
Figures
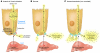
Comment on
-
Modulation of bone morphogenetic protein signaling in vivo regulates systemic iron balance.J Clin Invest. 2007 Jul;117(7):1933-9. doi: 10.1172/JCI31342. J Clin Invest. 2007. PMID: 17607365 Free PMC article.
References
-
- Nemeth E., Ganz T. Regulation of iron metabolism by hepcidin. Annu. Rev. Nutr. 2006;26:323–342. - PubMed
-
- Nemeth E., et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. - PubMed
-
- Pietrangelo A., et al. STAT3 is required for IL-6-gp130-dependent activation of hepcidin in vivo. Gastroenterology. 2007;132:294–300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

