Chain-reaction Ca(2+) signaling in the heart
- PMID: 17607353
- PMCID: PMC1904329
- DOI: 10.1172/JCI32496
Chain-reaction Ca(2+) signaling in the heart
Abstract
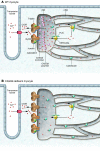
Mutations in Ca(2+) -handling proteins in the heart have been linked to exercise-induced sudden cardiac death. The best characterized of these have been mutations in the cardiac Ca(2+) release channel known as the ryanodine receptor type 2 (RyR2). RyR2 mutations cause "leaky" channels, resulting in diastolic Ca(2+) leak from the sarcoplasmic reticulum (SR) that can trigger fatal cardiac arrhythmias during stress. In this issue of the JCI, Song et al. show that mutations in the SR Ca(2+)-binding protein calsequestrin 2 (CASQ2) in mice result not only in reduced CASQ2 expression but also in a surprising, compensatory elevation in expression of both the Ca(2+)-binding protein calreticulin and RyR2, culminating in premature Ca(2+) release from cardiac myocytes and stress-induced arrhythmia (see the related article beginning on page 1814). In the context of these findings and other recent reports studying CASQ2 mutations, we discuss how CASQ2 influences the properties of Ca(2+)-dependent regulation of RyR2 and how this contributes to cardiac arrhythmogenesis.
Figures
Comment on
-
Calsequestrin 2 (CASQ2) mutations increase expression of calreticulin and ryanodine receptors, causing catecholaminergic polymorphic ventricular tachycardia.J Clin Invest. 2007 Jul;117(7):1814-23. doi: 10.1172/JCI31080. J Clin Invest. 2007. PMID: 17607358 Free PMC article.
Similar articles
-
Calsequestrin 2 (CASQ2) mutations increase expression of calreticulin and ryanodine receptors, causing catecholaminergic polymorphic ventricular tachycardia.J Clin Invest. 2007 Jul;117(7):1814-23. doi: 10.1172/JCI31080. J Clin Invest. 2007. PMID: 17607358 Free PMC article.
-
Modest reductions of cardiac calsequestrin increase sarcoplasmic reticulum Ca2+ leak independent of luminal Ca2+ and trigger ventricular arrhythmias in mice.Circ Res. 2007 Sep 14;101(6):617-26. doi: 10.1161/CIRCRESAHA.107.157552. Epub 2007 Jul 26. Circ Res. 2007. PMID: 17656677
-
Catecholaminergic polymorphic ventricular tachycardia: recent mechanistic insights.Cardiovasc Res. 2005 Aug 15;67(3):379-87. doi: 10.1016/j.cardiores.2005.04.027. Cardiovasc Res. 2005. PMID: 15913575 Review.
-
Abnormal interactions of calsequestrin with the ryanodine receptor calcium release channel complex linked to exercise-induced sudden cardiac death.Circ Res. 2006 May 12;98(9):1151-8. doi: 10.1161/01.RES.0000220647.93982.08. Epub 2006 Apr 6. Circ Res. 2006. PMID: 16601229
-
Modulation of ryanodine receptor by luminal calcium and accessory proteins in health and cardiac disease.Cardiovasc Res. 2008 Jan 15;77(2):245-55. doi: 10.1093/cvr/cvm038. Epub 2007 Oct 15. Cardiovasc Res. 2008. PMID: 18006456 Review.
Cited by
-
Functional characterization of CaVα2δ mutations associated with sudden cardiac death.J Biol Chem. 2015 Jan 30;290(5):2854-69. doi: 10.1074/jbc.M114.597930. Epub 2014 Dec 19. J Biol Chem. 2015. PMID: 25527503 Free PMC article.
-
Subcellular Ca2+ signaling in the heart: the role of ryanodine receptor sensitivity.J Gen Physiol. 2010 Aug;136(2):135-42. doi: 10.1085/jgp.201010406. J Gen Physiol. 2010. PMID: 20660656 Free PMC article. No abstract available.
-
Store-dependent deactivation: cooling the chain-reaction of myocardial calcium signaling.J Mol Cell Cardiol. 2013 May;58:77-83. doi: 10.1016/j.yjmcc.2012.10.008. Epub 2012 Oct 27. J Mol Cell Cardiol. 2013. PMID: 23108187 Free PMC article. Review.
-
Minding the calcium store: Ryanodine receptor activation as a convergent mechanism of PCB toxicity.Pharmacol Ther. 2010 Feb;125(2):260-85. doi: 10.1016/j.pharmthera.2009.10.009. Epub 2009 Nov 25. Pharmacol Ther. 2010. PMID: 19931307 Free PMC article. Review.
-
Sildenafil and FDP-Sr attenuate diabetic cardiomyopathy by suppressing abnormal expression of myocardial CASQ2, FKBP12.6, and SERCA2a in rats.Acta Pharmacol Sin. 2011 Apr;32(4):441-8. doi: 10.1038/aps.2010.226. Epub 2011 Mar 28. Acta Pharmacol Sin. 2011. PMID: 21441944 Free PMC article.
References
-
- Cheng H., Lederer W.J., Cannell M.B. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. - PubMed
-
- Shannon T.R., Guo T., Bers D.M. Ca2+ scraps: local depletions of free [Ca2+] in cardiac sarcoplasmic reticulum during contractions leave substantial Ca2+ reserve. Circ. Res. 2003;93:40–45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

