Priming and effector dependence on insulin B:9-23 peptide in NOD islet autoimmunity
- PMID: 17607359
- PMCID: PMC1904318
- DOI: 10.1172/JCI31368
Priming and effector dependence on insulin B:9-23 peptide in NOD islet autoimmunity
Abstract
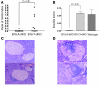
NOD mice with knockout of both native insulin genes and a mutated proinsulin transgene, alanine at position B16 in preproinsulin (B16:A-dKO mice), do not develop diabetes. Transplantation of NOD islets, but not bone marrow, expressing native insulin sequences (tyrosine at position B16) into B16:A-dKO mice rapidly restored development of insulin autoantibodies (IAAs) and insulitis, despite the recipients' pancreatic islets lacking native insulin sequences. Splenocytes from B16:A-dKO mice that received native insulin-positive islets induced diabetes when transferred into wild-type NOD/SCID or B16:A-dKO NOD/SCID mice. Splenocytes from mice immunized with native insulin B chain amino acids 9-23 (insulin B:9-23) peptide in CFA induced rapid diabetes upon transfer only in recipients expressing the native insulin B:9-23 sequence in their pancreata. Additionally, CD4(+) T cells from B16:A-dKO mice immunized with native insulin B:9-23 peptide promoted IAAs in NOD/SCID mice. These results indicate that the provision of native insulin B:9-23 sequences is sufficient to prime anti-insulin autoimmunity and that subsequent transfer of diabetes following peptide immunization requires native insulin B:9-23 expression in islets. Our findings demonstrate dependence on B16 alanine versus tyrosine of insulin B:9-23 for both the initial priming and the effector phase of NOD anti-islet autoimmunity.
Figures
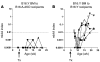



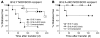



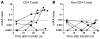
Similar articles
-
Long-term prevention of diabetes and marked suppression of insulin autoantibodies and insulitis in mice lacking native insulin B9-23 sequence.Ann N Y Acad Sci. 2006 Oct;1079:122-9. doi: 10.1196/annals.1375.018. Ann N Y Acad Sci. 2006. PMID: 17130542
-
Administration of a determinant of preproinsulin can induce regulatory T cells and suppress anti-islet autoimmunity in NOD mice.Clin Immunol. 2010 Jul;136(1):74-82. doi: 10.1016/j.clim.2010.02.016. Epub 2010 Apr 1. Clin Immunol. 2010. PMID: 20359955
-
Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice.Nature. 2005 May 12;435(7039):220-3. doi: 10.1038/nature03523. Nature. 2005. PMID: 15889095 Free PMC article.
-
Antigen presentation events during the initiation of autoimmune diabetes in the NOD mouse.J Autoimmun. 2016 Jul;71:19-25. doi: 10.1016/j.jaut.2016.03.007. Epub 2016 Mar 24. J Autoimmun. 2016. PMID: 27021276 Free PMC article. Review.
-
Identifying New Hybrid Insulin Peptides (HIPs) in Type 1 Diabetes.Front Immunol. 2021 Apr 30;12:667870. doi: 10.3389/fimmu.2021.667870. eCollection 2021. Front Immunol. 2021. PMID: 33995402 Free PMC article. Review.
Cited by
-
B-cell cross-presentation of autologous antigen precipitates diabetes.Diabetes. 2012 Nov;61(11):2893-905. doi: 10.2337/db12-0006. Epub 2012 Jul 24. Diabetes. 2012. PMID: 22829452 Free PMC article.
-
Subcutaneous insulin B:9-23/IFA immunisation induces Tregs that control late-stage prediabetes in NOD mice through IL-10 and IFNgamma.Diabetologia. 2010 Sep;53(9):1958-70. doi: 10.1007/s00125-010-1777-x. Epub 2010 May 20. Diabetologia. 2010. PMID: 20490452 Free PMC article.
-
Banting Lecture 2009: An unfinished journey: molecular pathogenesis to prevention of type 1A diabetes.Diabetes. 2010 Apr;59(4):759-74. doi: 10.2337/db09-1855. Diabetes. 2010. PMID: 20350969 Free PMC article.
-
Tracking of an Oral Salmonella-Based Vaccine for Type 1 Diabetes in Non-obese Diabetic Mice.Front Immunol. 2020 Apr 28;11:712. doi: 10.3389/fimmu.2020.00712. eCollection 2020. Front Immunol. 2020. PMID: 32411136 Free PMC article.
-
Exploring the Triple Interaction between the Host Genome, the Epigenome, and the Gut Microbiome in Type 1 Diabetes.Int J Mol Sci. 2020 Dec 24;22(1):125. doi: 10.3390/ijms22010125. Int J Mol Sci. 2020. PMID: 33374418 Free PMC article. Review.
References
-
- Todd J.A., Bell J.I., McDevitt H.O. HLA-DQB gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature. 1987;329:599–604. - PubMed
-
- Corper A.L., et al. A structural framework for deciphering the link between I-Ag7 and autoimmune diabetes. Science. 2000;288:505–511. - PubMed
-
- Narendran P., Mannering S.I., Harrison L.C. Proinsulin — a pathogenic autoantigen in type 1 diabetes. Autoimmun. Rev. 2003;2:204–210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

