Modulation of prostate cancer genetic risk by omega-3 and omega-6 fatty acids
- PMID: 17607361
- PMCID: PMC1890998
- DOI: 10.1172/JCI31494
Modulation of prostate cancer genetic risk by omega-3 and omega-6 fatty acids
Abstract
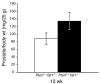
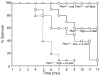
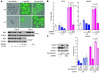
Although a causal role of genetic alterations in human cancer is well established, it is still unclear whether dietary fat can modulate cancer risk in a predisposed population. Epidemiological studies suggest that diets rich in omega-3 polyunsaturated fatty acids reduce cancer incidence. To determine the influence of fatty acids on prostate cancer risk in animals with a defined genetic lesion, we used prostate-specific Pten-knockout mice, an immune-competent, orthotopic prostate cancer model, and diets with defined polyunsaturated fatty acid levels. We found that omega-3 fatty acids reduced prostate tumor growth, slowed histopathological progression, and increased survival, whereas omega-6 fatty acids had opposite effects. Introducing an omega-3 desaturase, which converts omega-6 to omega-3 fatty acids, into the Pten-knockout mice reduced tumor growth similarly to the omega-3 diet. Tumors from mice on the omega-3 diet had lower proportions of phosphorylated Bad and higher apoptotic indexes compared with those from mice on omega-6 diet. Knockdown of Bad eliminated omega-3-induced cell death, and introduction of exogenous Bad restored the sensitivity to omega-3 fatty acids. Our data suggest that modulation of prostate cancer development by polyunsaturated fatty acids is mediated in part through Bad-dependent apoptosis. This study highlights the importance of gene-diet interactions in prostate cancer.
Figures



Similar articles
-
Effect of dietary polyunsaturated fatty acids on castration-resistant Pten-null prostate cancer.Carcinogenesis. 2012 Feb;33(2):404-12. doi: 10.1093/carcin/bgr290. Epub 2011 Dec 8. Carcinogenesis. 2012. PMID: 22159221 Free PMC article.
-
Effect of altering dietary omega-6/omega-3 fatty acid ratios on prostate cancer membrane composition, cyclooxygenase-2, and prostaglandin E2.Clin Cancer Res. 2006 Aug 1;12(15):4662-70. doi: 10.1158/1078-0432.CCR-06-0459. Clin Cancer Res. 2006. PMID: 16899616 Free PMC article.
-
A low dietary ratio of omega-6 to omega-3 Fatty acids may delay progression of prostate cancer.Nutr Cancer. 2013;65(4):556-62. doi: 10.1080/01635581.2013.775316. Nutr Cancer. 2013. PMID: 23659447
-
Genetic variants in the metabolism of omega-6 and omega-3 fatty acids: their role in the determination of nutritional requirements and chronic disease risk.Exp Biol Med (Maywood). 2010 Jul;235(7):785-95. doi: 10.1258/ebm.2010.009298. Exp Biol Med (Maywood). 2010. PMID: 20558833 Review.
-
The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases.Exp Biol Med (Maywood). 2008 Jun;233(6):674-88. doi: 10.3181/0711-MR-311. Epub 2008 Apr 11. Exp Biol Med (Maywood). 2008. PMID: 18408140 Review.
Cited by
-
Inhibiting delta-6 desaturase activity suppresses tumor growth in mice.PLoS One. 2012;7(10):e47567. doi: 10.1371/journal.pone.0047567. Epub 2012 Oct 24. PLoS One. 2012. PMID: 23112819 Free PMC article.
-
Mechanisms of omega-3 polyunsaturated fatty acids in prostate cancer prevention.Biomed Res Int. 2013;2013:824563. doi: 10.1155/2013/824563. Epub 2013 May 23. Biomed Res Int. 2013. PMID: 23762859 Free PMC article. Review.
-
Growth of human gastric cancer cells in nude mice is delayed by a ketogenic diet supplemented with omega-3 fatty acids and medium-chain triglycerides.BMC Cancer. 2008 Apr 30;8:122. doi: 10.1186/1471-2407-8-122. BMC Cancer. 2008. PMID: 18447912 Free PMC article.
-
In vivo and in vitro regulation of syndecan 1 in prostate cells by n-3 polyunsaturated fatty acids.J Biol Chem. 2008 Jun 27;283(26):18441-9. doi: 10.1074/jbc.M802107200. Epub 2008 Apr 30. J Biol Chem. 2008. PMID: 18450755 Free PMC article.
-
Effects of dietary fish oil on the depletion of carcinogenic PAH-DNA adduct levels in the liver of B6C3F1 mouse.PLoS One. 2011;6(10):e26589. doi: 10.1371/journal.pone.0026589. Epub 2011 Oct 31. PLoS One. 2011. PMID: 22066002 Free PMC article.
References
-
- Kolonel L.N. Fat, meat, and prostate cancer. Epidemiol. Rev. 2001;23:72–81. - PubMed
-
- Wynder E.L., Mabuchi K., Whitmore W.F., Jr. Epidemiology of cancer of the prostate. Cancer. 1971;28:344–360. - PubMed
-
- Breslow N., et al. Latent carcinoma of prostate at autopsy in seven areas. The International Agency for Research on Cancer. Lyon, France. Int. J. Cancer. 1977;20:680–688. - PubMed
-
- Dunn J.E. Cancer epidemiology in populations of the United States — with emphasis on Hawaii and California — and Japan. Cancer Res. 1975;35:3240–3245. - PubMed
-
- Haenszel W., Kurihara M. Studies of Japanese migrants. I. Mortality from cancer and other diseases among Japanese in the United States. J. Natl. Cancer Inst. 1968;40:43–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

