Energy and electron transfer in enhanced two-photon-absorbing systems with triplet cores
- PMID: 17608457
- PMCID: PMC2441487
- DOI: 10.1021/jp071586f
Energy and electron transfer in enhanced two-photon-absorbing systems with triplet cores
Abstract
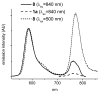
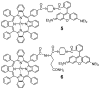
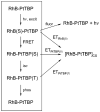
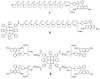
Enhanced two-photon-absorbing (2PA) systems with triplet cores are currently under scrutiny for several biomedical applications, including photodynamic therapy (PDT) and two-photon microscopy of oxygen. The performance of so far developed molecules, however, is substantially below expected. In this study we take a detailed look at the processes occurring in these systems and propose ways to improve their performance. We focus on the interchromophore distance tuning as a means for optimization of two-photon sensors for oxygen. In these constructs, energy transfer from several 2PA chromophores is used to enhance the effective 2PA cross section of phosphorescent metalloporphyrins. Previous studies have indicated that intramolecular electron transfer (ET) can act as an effective quencher of phosphorescence, decreasing the overall sensor efficiency. We studied the interplay between 2PA, energy transfer, electron transfer, and phosphorescence emission using Rhodamine B-Pt tetrabenzoporphyrin (RhB-PtTBP) adducts as model compounds. 2PA cross sections (sigma2) of tetrabenzoporphyrins (TBPs) are in the range of several tens of GM units (near 800 nm), making TBPs superior 2PA chromophores compared to regular porphyrins (sigma2 values typically 1-2 GM). Relatively large 2PA cross sections of rhodamines (about 200 GM in 800-850 nm range) and their high photostabilities make them good candidates as 2PA antennae. Fluorescence of Rhodamine B (lambda(fl) = 590 nm, phi(fl) = 0.5 in EtOH) overlaps with the Q-band of phosphorescent PtTBP (lambda(abs) = 615 nm, epsilon = 98 000 M(-1) cm(-1), phi(p) approximately 0.1), suggesting that a significant amplification of the 2PA-induced phosphorescence via fluorescence resonance energy transfer (FRET) might occur. However, most of the excitation energy in RhB-PtTBP assemblies is consumed in several intramolecular ET processes. By installing rigid nonconducting decaproline spacers (Pro10) between RhB and PtTBP, the intramolecular ETs were suppressed, while the chromophores were kept within the Förster r0 distance in order to maintain high FRET efficiency. The resulting assemblies exhibit linear amplification of their 2PA-induced phosphorescence upon increase in the number of 2PA antenna chromophores and show high oxygen sensitivity. We also have found that PtTBPs possess unexpectedly strong forbidden S0 --> T1 bands (lambda(max) = 762 nm, epsilon = 120 M-1 cm-1). The latter may overlap with the laser spectrum and lead to unwanted linear excitation.
Figures
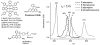
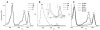

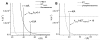

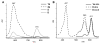


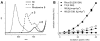
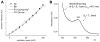
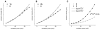
References
-
- The quantity σ2 is commonly referred to as the “two-photon-absorption cross section,” although it is not an area (see, for example: www.drbio.cornell.edu/MPE/mpe.html). It is the proportionality coefficient between the photon absorption rate per molecule (β) and the time-averaged square of the photon flux Φ: β = σ2〈Φ2〉. σ2 is measured in GM (Göppert-Mayer) units: 1 GM = 10-50 cm4 s photon-1.
-
- Denk W, Strickler JH, Webb WW. Science. 1990;248:73–76. - PubMed
-
- Svoboda K, Yasuda R. Neuron. 2006;50:823–839. For recent reviews, see. - PubMed
- Rothstein EC, Nauman M, Chesnick S, Balaban RS. J. Microsc. (Oxford) 2006;222:58–64. - PMC - PubMed
- Helmchen F, Denk W. Nat. Methods. 2005;2:932–940. - PubMed
- Helmchen F, Denk W. Curr. Opin. Neurobiol. 2002;12:593–601. - PubMed
-
- Göppert-Mayer M. Ann. Phys. 1931;9:273.
-
- Parthenopoulos DA, Rentzepis PM. Science. 1989;245:843–845. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

