Rb-mediated neuronal differentiation through cell-cycle-independent regulation of E2f3a
- PMID: 17608565
- PMCID: PMC1914394
- DOI: 10.1371/journal.pbio.0050179
Rb-mediated neuronal differentiation through cell-cycle-independent regulation of E2f3a
Abstract
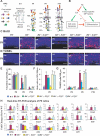
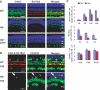
It has long been known that loss of the retinoblastoma protein (Rb) perturbs neural differentiation, but the underlying mechanism has never been solved. Rb absence impairs cell cycle exit and triggers death of some neurons, so differentiation defects may well be indirect. Indeed, we show that abnormalities in both differentiation and light-evoked electrophysiological responses in Rb-deficient retinal cells are rescued when ectopic division and apoptosis are blocked specifically by deleting E2f transcription factor (E2f) 1. However, comprehensive cell-type analysis of the rescued double-null retina exposed cell-cycle-independent differentiation defects specifically in starburst amacrine cells (SACs), cholinergic interneurons critical in direction selectivity and developmentally important rhythmic bursts. Typically, Rb is thought to block division by repressing E2fs, but to promote differentiation by potentiating tissue-specific factors. Remarkably, however, Rb promotes SAC differentiation by inhibiting E2f3 activity. Two E2f3 isoforms exist, and we find both in the developing retina, although intriguingly they show distinct subcellular distribution. E2f3b is thought to mediate Rb function in quiescent cells. However, in what is to our knowledge the first work to dissect E2f isoform function in vivo we show that Rb promotes SAC differentiation through E2f3a. These data reveal a mechanism through which Rb regulates neural differentiation directly, and, unexpectedly, it involves inhibition of E2f3a, not potentiation of tissue-specific factors.
Conflict of interest statement
Figures





Similar articles
-
Rb is required for retinal angiogenesis and lamination.Cell Death Dis. 2018 Mar 6;9(3):370. doi: 10.1038/s41419-018-0411-6. Cell Death Dis. 2018. PMID: 29511172 Free PMC article.
-
E2f2 induces cone photoreceptor apoptosis independent of E2f1 and E2f3.Cell Death Differ. 2013 Jul;20(7):931-40. doi: 10.1038/cdd.2013.24. Epub 2013 Apr 5. Cell Death Differ. 2013. PMID: 23558950 Free PMC article.
-
E2f1-3 switch from activators in progenitor cells to repressors in differentiating cells.Nature. 2009 Dec 17;462(7275):930-4. doi: 10.1038/nature08677. Nature. 2009. PMID: 20016602 Free PMC article.
-
Retinoblastoma-E2F Transcription Factor Interplay Is Essential for Testicular Development and Male Fertility.Front Endocrinol (Lausanne). 2022 May 19;13:903684. doi: 10.3389/fendo.2022.903684. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35663332 Free PMC article. Review.
-
The RB protein family in retinal development and retinoblastoma: new insights from new mouse models.Dev Neurosci. 2004;26(5-6):417-34. doi: 10.1159/000082284. Dev Neurosci. 2004. PMID: 15855771 Review.
Cited by
-
Rb is required for retinal angiogenesis and lamination.Cell Death Dis. 2018 Mar 6;9(3):370. doi: 10.1038/s41419-018-0411-6. Cell Death Dis. 2018. PMID: 29511172 Free PMC article.
-
Loss of MAP3K1 enhances proliferation and apoptosis during retinal development.Development. 2011 Sep;138(18):4001-12. doi: 10.1242/dev.065003. Development. 2011. PMID: 21862560 Free PMC article.
-
The gut microbiota regulates diabetic retinopathy in adult rats.Front Microbiol. 2025 Jan 29;16:1479792. doi: 10.3389/fmicb.2025.1479792. eCollection 2025. Front Microbiol. 2025. PMID: 39949626 Free PMC article.
-
Genome-wide analysis reveals NRP1 as a direct HIF1α-E2F7 target in the regulation of motorneuron guidance in vivo.Nucleic Acids Res. 2016 May 5;44(8):3549-66. doi: 10.1093/nar/gkv1471. Epub 2015 Dec 17. Nucleic Acids Res. 2016. PMID: 26681691 Free PMC article.
-
E2f2 induces cone photoreceptor apoptosis independent of E2f1 and E2f3.Cell Death Differ. 2013 Jul;20(7):931-40. doi: 10.1038/cdd.2013.24. Epub 2013 Apr 5. Cell Death Differ. 2013. PMID: 23558950 Free PMC article.
References
-
- Dyer MA, Bremner R. The search for the retinoblastoma cell of origin. Nat Rev Cancer. 2005;5:91–101. - PubMed
-
- Chen D, Livne-Bar I, Vanderluit JL, Slack RS, Agochiya M, et al. Cell-specific effects of RB or RB/p107 loss on retinal development implicate an intrinsically death-resistant cell-of-origin in retinoblastoma. Cancer Cell. 2004;5:539–551. - PubMed
-
- Stevaux O, Dyson NJ. A revised picture of the E2F transcriptional network and RB function. Curr Opin Cell Biol. 2002;14:684–691. - PubMed
-
- Wu L, Timmers C, Maiti B, Saavedra HI, Sang L, et al. The E2F1–3 transcription factors are essential for cellular proliferation. Nature. 2001;414:457–462. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

