Structural basis of PP2A inhibition by small t antigen
- PMID: 17608567
- PMCID: PMC1945078
- DOI: 10.1371/journal.pbio.0050202
Structural basis of PP2A inhibition by small t antigen
Abstract
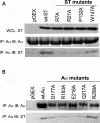
The SV40 small t antigen (ST) is a potent oncoprotein that perturbs the function of protein phosphatase 2A (PP2A). ST directly interacts with the PP2A scaffolding A subunit and alters PP2A activity by displacing regulatory B subunits from the A subunit. We have determined the crystal structure of full-length ST in complex with PP2A A subunit at 3.1 A resolution. ST consists of an N-terminal J domain and a C-terminal unique domain that contains two zinc-binding motifs. Both the J domain and second zinc-binding motif interact with the intra-HEAT-repeat loops of HEAT repeats 3-7 of the A subunit, which overlaps with the binding site of the PP2A B56 subunit. Intriguingly, the first zinc-binding motif is in a position that may allow it to directly interact with and inhibit the phosphatase activity of the PP2A catalytic C subunit. These observations provide a structural basis for understanding the oncogenic functions of ST.
Conflict of interest statement
Figures




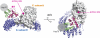

Similar articles
-
Structural and biochemical insights into the regulation of protein phosphatase 2A by small t antigen of SV40.Nat Struct Mol Biol. 2007 Jun;14(6):527-34. doi: 10.1038/nsmb1254. Epub 2007 May 27. Nat Struct Mol Biol. 2007. PMID: 17529992
-
The structure of the PP2A regulatory subunit B56 gamma: the remaining piece of the PP2A jigsaw puzzle.Proteins. 2009 Jan;74(1):212-21. doi: 10.1002/prot.22150. Proteins. 2009. PMID: 18618707
-
Restricted protein phosphatase 2A targeting by Merkel cell polyomavirus small T antigen.J Virol. 2015 Apr;89(8):4191-200. doi: 10.1128/JVI.00157-15. Epub 2015 Jan 28. J Virol. 2015. PMID: 25631078 Free PMC article.
-
PP2A holoenzyme assembly: in cauda venenum (the sting is in the tail).Trends Biochem Sci. 2008 Mar;33(3):113-21. doi: 10.1016/j.tibs.2007.12.004. Trends Biochem Sci. 2008. PMID: 18291659 Review.
-
PP2A fulfills its promises as tumor suppressor: which subunits are important?Cancer Cell. 2004 Feb;5(2):105-6. doi: 10.1016/s1535-6108(04)00027-3. Cancer Cell. 2004. PMID: 14998482 Review.
Cited by
-
Fifty Years of JC Polyomavirus: A Brief Overview and Remaining Questions.Viruses. 2020 Sep 1;12(9):969. doi: 10.3390/v12090969. Viruses. 2020. PMID: 32882975 Free PMC article. Review.
-
The oncogenic potential of BK-polyomavirus is linked to viral integration into the human genome.J Pathol. 2015 Nov;237(3):379-89. doi: 10.1002/path.4584. Epub 2015 Aug 19. J Pathol. 2015. PMID: 26172456 Free PMC article.
-
Protein phosphatase 2A controls ongoing DNA replication by binding to and regulating cell division cycle 45 (CDC45).J Biol Chem. 2019 Nov 8;294(45):17043-17059. doi: 10.1074/jbc.RA119.010432. Epub 2019 Sep 27. J Biol Chem. 2019. PMID: 31562245 Free PMC article.
-
Molecular dissection on inhibition of Ras-induced cellular senescence by small t antigen of SV40.Cell Mol Life Sci. 2022 Apr 16;79(5):242. doi: 10.1007/s00018-022-04275-5. Cell Mol Life Sci. 2022. PMID: 35429286 Free PMC article.
-
Defect of insulin signal in peripheral tissues: Important role of ceramide.World J Diabetes. 2014 Jun 15;5(3):244-57. doi: 10.4239/wjd.v5.i3.244. World J Diabetes. 2014. PMID: 24936246 Free PMC article. Review.
References
-
- Barbanti-Brodano G, Sabbioni S, Martini F, Negrini M, Corallini A, et al. Simian virus 40 infection in humans and association with human diseases: Results and hypotheses. Virology. 2004;318:1–9. - PubMed
-
- Skoczylas C, Fahrbach KM, Rundell K. Cellular targets of the SV40 small-t antigen in human cell transformation. Cell Cycle. 2004;3:606–610. - PubMed
-
- Rundell K, Parakati R. The role of the SV40 ST antigen in cell growth promotion and transformation. Semin Cancer Biol. 2001;11:5–13. - PubMed
-
- Yu J, Boyapati A, Rundell K. Critical role for SV40 small-t antigen in human cell transformation. Virology. 2001;290:192–198. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

