Regions important for the adhesin activity of Moraxella catarrhalis Hag
- PMID: 17608944
- PMCID: PMC1931440
- DOI: 10.1186/1471-2180-7-65
Regions important for the adhesin activity of Moraxella catarrhalis Hag
Abstract
Background: The Moraxella catarrhalis Hag protein, an Oca autotransporter adhesin, has previously been shown to be important for adherence of this respiratory tract pathogen to human middle ear and A549 lung cells.
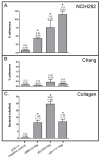
Results: The present study demonstrates that adherence of M. catarrhalis isogenic hag mutant strains to the human epithelial cell lines Chang (conjunctival) and NCIH292 (lung) is reduced by 50-93%. Furthermore, expressing Hag in a heterologous Escherichia coli background substantially increased the adherence of recombinant bacteria to NCIH292 cells and murine type IV collagen. Hag did not, however, increase the attachment of E. coli to Chang cells. These results indicate that Hag directly mediates adherence to NCIH292 lung cells and collagen, but is not sufficient to confer binding to conjunctival monolayers. Several in-frame deletions were engineered within the hag gene of M. catarrhalis strain O35E and the resulting proteins were tested for their ability to mediate binding to NCIH292 monolayers, middle ear cells, and type IV collagen. These experiments revealed that epithelial cell and collagen binding properties are separable, and that residues 385-705 of this ~2,000 amino acid protein are important for adherence to middle ear and NCIH292 cells. The region of O35E-Hag encompassing aa 706 to 1194 was also found to be required for adherence to collagen. In contrast, beta-roll repeats present in Hag, which are structural features conserved in several Oca adhesins and responsible for the adhesive properties of Yersinia enterocolitica YadA, are not important for Hag-mediated adherence.
Conclusion: Hag is a major adherence factor for human cells derived from various anatomical sites relevant to pathogenesis by M. catarrhalis and its structure-function relationships differ from those of other, closely-related autotransporter proteins.
Figures
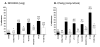



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

