Human T-cell leukemia virus type 1 (HTLV-1) p12I down-modulates ICAM-1 and -2 and reduces adherence of natural killer cells, thereby protecting HTLV-1-infected primary CD4+ T cells from autologous natural killer cell-mediated cytotoxicity despite the reduction of major histocompatibility complex class I molecules on infected cells
- PMID: 17609265
- PMCID: PMC2045425
- DOI: 10.1128/JVI.00887-07
Human T-cell leukemia virus type 1 (HTLV-1) p12I down-modulates ICAM-1 and -2 and reduces adherence of natural killer cells, thereby protecting HTLV-1-infected primary CD4+ T cells from autologous natural killer cell-mediated cytotoxicity despite the reduction of major histocompatibility complex class I molecules on infected cells
Abstract
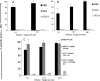
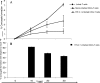
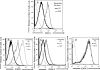
Although natural killer (NK) cell-mediated control of viral infections is well documented, very little is known about the ability of NK cells to restrain human T-cell leukemia virus type 1 (HTLV-1) infection. In the current study we show that NK cells are unable to kill HTLV-1-infected primary CD4+ T cells. Exposure of NK cells to interleukin-2 (IL-2) resulted in only a marginal increase in their ability to kill HTLV-1-infected primary CD4+ T cells. This inability of NK cells to kill HTLV-1-infected CD4+ T cells occurred despite the down-modulation of major histocompatibility complex (MHC) class I molecules, one of the ligands for the major NK cell inhibitory receptor, by HTLV-1 p12(I) on CD4+ T cells. One reason for this diminished ability of NK cells to kill HTLV-1-infected cells was the decreased ability of NK cells to adhere to HTLV-1-infected cells because of HTLV-1 p12(I)-mediated down-modulation of intercellular adhesion molecule 1 (ICAM-1) and ICAM-2. We also found that HTLV-1-infected CD4+ T cells did not express ligands for NK cell activating receptors, NCR and NKG2D, although they did express ligands for NK cell coactivating receptors, NTB-A and 2B4. Thus, despite HTLV-1-mediated down-modulation of MHC-I molecules, HTLV-1-infected primary CD4+ T cells avoids NK cell destruction by modulating ICAM expression and shunning the expression of ligands for activating receptors.
Figures



References
-
- Arnon, T. I., M. Lev, G. Katz, Y. Chernobrov, A. Porgador, and O. Mandelboim. 2001. Recognition of viral hemagglutinins by NKp44 but not by NKp30. Eur. J. Immunol. 31:2680-2689. - PubMed
-
- Bangham, C. R., and M. Osame. 2005. Cellular immune response to HTLV-1. Oncogene 24:6035-6046. - PubMed
-
- Barber, D. F., M. Faure, and E. O. Long. 2004. LFA-1 contributes an early signal for NK cell cytotoxicity. J. Immunol. 173:3653-3659. - PubMed
-
- Barber, D. F., and E. O. Long. 2003. Coexpression of CD58 or CD48 with intercellular adhesion molecule 1 on target cells enhances adhesion of resting NK cells. J. Immunol. 170:294-299. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

