Alpha interferon enhances TRIM5alpha-mediated antiviral activities in human and rhesus monkey cells
- PMID: 17609277
- PMCID: PMC2045407
- DOI: 10.1128/JVI.00419-07
Alpha interferon enhances TRIM5alpha-mediated antiviral activities in human and rhesus monkey cells
Abstract
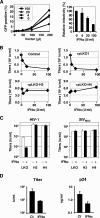
Dominant, constitutively expressed antiretroviral factors, including TRIM5alpha and APOBEC3 proteins, are distinguished from the conventional innate immune systems and are classified as intrinsic immunity factors. Here, we demonstrate that interferon alpha (IFN-alpha) treatment upregulates TRIM5alpha mRNA in rhesus monkey cells, which correlates with the enhanced TRIM5alpha-mediated pre- and postintegration blocks of human immunodeficiency virus replication. In human cells, IFN-alpha increases the levels of TRIM5alpha mRNA, resulting in enhanced antiviral activity against N-tropic murine leukemia virus infection. These observations indicate that the TRIM5alpha-mediated antiviral effects can be orchestrated by the conventional innate immune response. It is conceivable that TRIM5alpha plays an essential role in controlling both the initial retroviral exposure and the subsequent viral dissemination in vivo.
Figures


References
-
- Abudu, A., A. Takaori-Kondo, T. Izumi, K. Shirakawa, M. Kobayashi, A. Sasada, K. Fukunaga, and T. Uchiyama. 2006. Murine retrovirus escapes from murine APOBEC3 via two distinct novel mechanisms. Curr. Biol. 16:1565-1570. - PubMed
-
- Asaoka, K., K. Ikeda, T. Hishinuma, K. Horie-Inoue, S. Takeda, and S. Inoue. 2005. A retrovirus restriction factor TRIM5alpha is transcriptionally regulated by interferons. Biochem. Biophys. Res. Commun. 338:1950-1956. - PubMed
-
- Best, S., P. Le Tissier, G. Towers, and J. P. Stoye. 1996. Positional cloning of the mouse retrovirus restriction gene Fv1. Nature 382:826-829. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

